Table 2.
SAR of 5,6 fused ring heterocycles
| Compound | Structurea | mGlu5 IC50 (nM)b | % Glu Maxc | Synthetic Conditions |
|---|---|---|---|---|
| 14 |
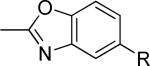
|
>30,000 | – | i |
| 15 |
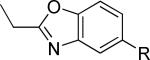
|
2460 ± 226 | 26.3 ± 14.4 | iii |
| 16 |
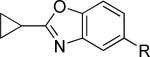
|
>10,000d | 46.5 ± 7.8 | i |
| 17 |
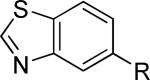
|
>30,000 | – | i |
| 18 |
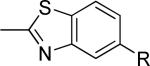
|
61 ± 7 | 0.63 ± 0.12 | i |
| 19 |
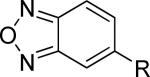
|
>30,000 | – | iii |
| 20 |
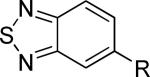
|
>30,000 | – | iii |
| 21 |
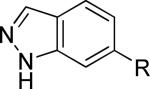
|
>10,000d | 60.4 ± 2.5 | iii |
| 22 |
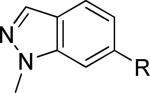
|
>30,000 | – | from 21e |
| 23 |
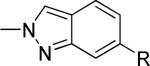
|
1520 ± 296 | 4.8 ± 2.3 | from 21e |
R = 3-cyano-5-fluorophenyl
Calcium mobilization mGlu5 assay; values are average of n ≥ 3
Amplitude of response in the presence of 30 μM test compound as a percentage of maximal response (100 μM glutamate); values are average of n ≥ 3
CRC does not plateau
Reaction of 21 with K2CO3 and MeI in DMF afforded a separable mixture of 22 and 23
