Abstract
Dorsal root ganglia (DRG) contain somatosensory neurons of diverse sensory modalities. Among these different types of sensory neurons, the molecular mechanisms that regulate the development and specification of touch neurons are least well understood. We took a candidate-approach and searched for transcription factors that are expressed in subsets of DRG neurons, and found that the transcription factor Shox2 is expressed in subpopulations of TrkB- and Ret-expressing neurons at neonatal stages. Since TrkB is a known marker selectively expressed in touch sensory neurons, we decided to examine the function of Shox2 in specifying TrkB-positive DRG neurons. Conditional deletion of Shox2 in neural crest cells (which give rise to all DRG neurons) caused a 60~65% reduction in the number of TrkB-expressing neurons. It also resulted in an increase in co-expression of TrkC in Ret-positive sensory neuron. Deletion of Shox2 in differentiating DRG neurons at later time points caused only a moderate reduction in TrkB expression. Overexpression of Shox2 in all neural crest cells resulted in a small increase in the number of TrkB-expressing neurons. Finally, Shox2-deletion also caused reduced touch sensory axonal innervation to layers III/IV of the spinal cord. Taken together, our findings identify Shox2 as an essential but not sufficient component of the transcription programs required in neural progenitor cells for the proper specification of subsets of TrkB-expressing touch/mechanosensory neurons.
Introduction
Somatosensory neurons located in dorsal root ganglia (DRG) consist of many different types that detect diverse modalities of sensory stimuli. All DRG neurons are originated from neural crest cells (NCCs) (Chai et al., 2000; Ma et al., 1999; Szeder et al., 2003). We are interested in identifying molecular mechanisms that enable NCCs to differentiation into touch/mechanosensory neurons. Different DRG neurons have unique molecular compositions. Receptors for neurotrophic factors are among the best characterized markers for sensory neurons. It has been shown that TrkA and Ret receptors are mainly expressed in nociceptive and thermal sensory neurons (Chen et al., 2006; Kramer et al., 2006; Luo et al., 2007). TrkC is expressed primarily in proprioceptive neurons innervating the skeletal muscles (Hasegawa and Wang, 2008; Hippenmeyer et al., 2005; Inoue et al., 2007; Sedy et al., 2006). TrkB is expressed by a subpopulation of cutaneous low-threshold touch neurons (Gonzalez-Martinez et al., 2004; Perez-Pinera et al., 2008; Shimizu et al., 2007). An early born population of Ret-expressing neurons develops into rapid-adapting mechanosensory neurons (Bourane et al., 2009; Luo et al., 2009). Signaling through neurotrophic receptors is important for neuron survival, axon growth, innervation of central and peripheral targets, and proper differentiation into specialized and modality-specific sensors (da Silva and Wang, 2010; Marmigere and Ernfors, 2007).
Significant progresses have been made in elucidating the transcriptional programs specifying nociceptive and proprioceptive neurons. For example, the transcription factor Runx1 is essential for differentiation and diversification of nociceptive neurons into peptidergic and non-peptidergic lineages (Chen et al., 2006; Inoue et al., 2008; Kramer et al., 2006; Marmigere and Ernfors, 2007), whereas Runx3 and Er81 are important for the specification of TrkC-expressing proprioceptive sensory neurons (Hippenmeyer et al., 2005; Inoue et al., 2007; Kramer et al., 2006; Levanon et al., 2001). Recently, MafA was shown to be involved in the development of Ret-positive rapid-adapting mechanoreceptors (Bourane et al., 2009). However, the transcription factors that enable progenitor cells to differentiate into TrkB-expressing mechanosensory neurons remain unclear.
We searched for transcription factors expressed in a subset of DRG neurons and found the gene encoding Shox2 (Short Stature Homeobox 2), a homeobox transcription factor, is dynamically expressed during DRG development. The mouse Shox2 gene displays 99% and 73% similarity to human Shox2 and Shox, respectively. Mutations in human Shox cause short statue and Leri-Weill dyschondrosteosis (Binder, 2011; Marchini et al., 2007). Mice only have the Shox2 gene. Thus, it appears that mouse Shox2 assumes the functions of both human Shox and Shox2. Shox2 mutant mice show defects in bone, heart, and palate development (Blaschke et al., 2007; Cobb et al., 2006; Yu et al., 2005). However, the role of Shox2 in neuronal development has not been examined. Here we performed loss- and gain-of-function analyses to determine the role of Shox2 in DRG development. We discovered that Shox2 is important for the development of TrkB-expressing mechanosensory neurons.
Materials and Methods
Mice
Shox2flox/flox (Cobb et al., 2006), Wnt1-Cre (Danielian et al., 1998), AdvillinCre/+ (Zhou et al., 2010), Isl1Cre/+ (Srinivas et al., 2001; Yang et al., 2006) and AdvillinPLAP/+ (Hasegawa et al., 2007) mice are all previously described. RosaCAG-STOP-Shox2/+ mouse was generated by inserting a cassette of “chicken βActin promoter-LoxP-neo-polyA-LoxP-Shox2-polyA” into the Rosa26 locus via homologous recombination. Genotyping of the RosaCAG-STOP-Shox2/+ mice was performed by PCR. PCR primers were designed as follows: Rosa/01, 5-CACTTGCTCTCCCAAAGTCG-3; Rosa/02, 5-TAGTCTAACTCGCGACACTG-3; CAG/02, 5-GTTATGTAACGCGGAACTCC-3. The wild-type allele produces a 560 bp fragment with Rosa/01 and Rosa/02 primers, whereas the knock-in allele results in a 300 bp fragment with Rosa/01 and CAG/02 primers. Furthermore, primers were designed to specifically detect the Shox2 cDNA in the RosaCAG-STOP-Shox2 allele: RShox2/01, 5-GTGTCCCCTGAACTGAAGGA-3; and RShox2/02, 5-GCCTGAACCTGAAAGGACAA-3. The knock-in allele produces a 400 bp fragment using the RShox2/01 and RShox2/02 primers. All experiments were conducted according to protocols approved by The Duke University Institutional Animal Care and Use Committee.
In situ hybridization
The mouse cDNA fragments of the neurotrophic receptors and Shox2 were amplified by PCR with the antisense primers containing the T7 promoter sequence. In vitro transcription was then performed from the PCR-amplified template using T7 RNA polymerase (Roche, Indianapolis, IN) with Digoxigenin-UTP (Roche) for the synthesis of the antisense probes. In situ hybridization was performed according to standard methods (Hodge et al., 2007). Fluorescent two-color in situ hybridization was performed according to standard methods (Hasegawa and Wang, 2008).
Immunostaining
Immunostaining was performed according to standard methods (Hodge et al., 2007). Antibody used are: Alexa Fluor 488-conjugated IB4 (Molecular Probes/Invitrogen, Eugene, OR), Anti-Caspase 3 (active) (1:1000; R&D Systems, Minneapolis, MN), Anti-CGRP antibody (1:2000; Chemicon/Invitrogen), Anti-PGP9.5 antibody (UltraClone, Isle of Wight, UK), Anti-vGluT1 antibody (1:1000; Millipore, Billerica, MA), Alexa 488-labeled anti-rabbit IgG (1:400; Invitrogen), Alexa 488-labeled anti-guinea pig IgG (1:400; Invitrogen), and Cy3-labeled anti-rabbit IgG (1:400, Jackson Labs, Bar Harbor, MA).
Alkaline phosphatase staining
Alkaline phosphatase staining was performed according to standard methods (Hasegawa et al., 2007).
Quantification methods
For every developmental stage, at least 3 embryos/pups from 2 to 3 different litters were analyzed. In situ (or immuno) signal positive DRG neurons were counted from randomly selected sections. For counting TrkA or Ret positive neurons, N=20 randomly selected sections from each animal are counted; for counting Shox2, TrkC, TrkB or activated caspase-3 positive neurons, N=40~50 randomly selected sections from each animal are counted; for counting MafA, Runx3, or parvalbumin positive neurons, N=30~35 randomly selected sections from each animal are counted. On each section, the area of the DRG was measured using MetaMorph software. Number of cells per unit area is then calculated, and averaged over all embryos/animals. p values were calculated using Student’s t test. PLAP staining intensity (from 50 randomly selected sections of animals of 2 different litters) and vGluT1 staining intensities and areas (from 60 randomly selected sections of animals of 2 different litters) were measured using MetaMorph software and were set to artificial units. p values were calculated with Student’s t test.
Results
Shox2 expression pattern in the developing mouse DRG
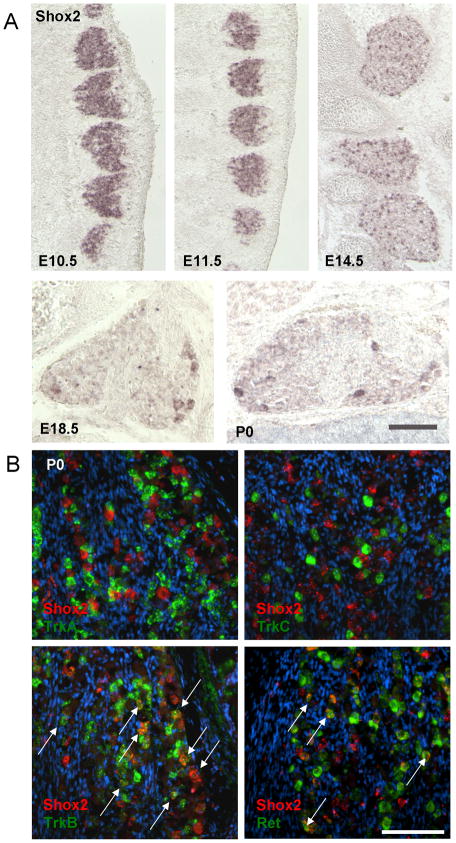
In situ hybridization was carried out to examine the expression of Shox2 in the developing mouse DRG. At early stages beginning at E10.5 (embryonic day 10.5), Shox2 is expressed throughout the ganglion (Figure 1A). By E14.5, its expression begins to down-regulate in the majority of sensory neurons (Figure 1A). This down regulation continues after E14.5 and by E18.5 only a small population of DRG neurons retain stable expression of Shox2, which persist into adulthood (Figure 1A and data not shown). The dynamic expression pattern of Shox2 led us to hypothesize that Shox2 is involved in the specification and development of subtypes of DRG neurons. To test this, we carried out fluorescent two-color in situ hybridization experiments to examine the potential co-expression of Shox2 with Trks or Ret receptors (which are markers for different somatosensory lineages). We observed that at P0 (postnatal day 0), Shox2 co-localizes with subsets of TrkB or Ret-expressing neurons and is absent from the TrkA- and TrkC-expressing cells (Figure 1B). Upon quantification, we found that 65% of Shox2-postive cells co-localize with 65% of TrkB-neurons, and the remaining 35% of Shox2-expressing cells co-localizes with a small number of Ret-positive DRG neurons. Since TrkB has been shown to be a marker for subsets of mechanosensory neurons, and Ret is expressed in some of the rapid-adapting mechanosensory neurons in addition to nociceptive neurons, the co-expression results suggest that Shox2 may be involved in the differentiation of NCCs into mechanosensory neurons.
Figure 1. Shox2 expression in the developing DRG.
A. In situ hybridization experiments show that at E10.5 and E11.5, Shox2 is expressed throughout the entire DRG; at E14.5, Shox2 expression begins to down-regulate in subsets of DRG neurons; and by E18.5 and P0, Shox2 is only present in a small number of DRG neurons. B. Two color in situ hybridization with probes for Shox2 (red) and different receptors (TrkA, TrkB, TrkC and Ret) (green). Arrows indicate cell bodies in the ganglia showing co-localization of Shox2 with either TrkB or Ret. Note that not all TrkB or Ret positive cell bodies have Shox2 expression at P0. Scale bar = 100um
Significant reduction in the number of TrkB-expressing DRG neurons in Wnt1-Cre; Shox2flox/flox mouse
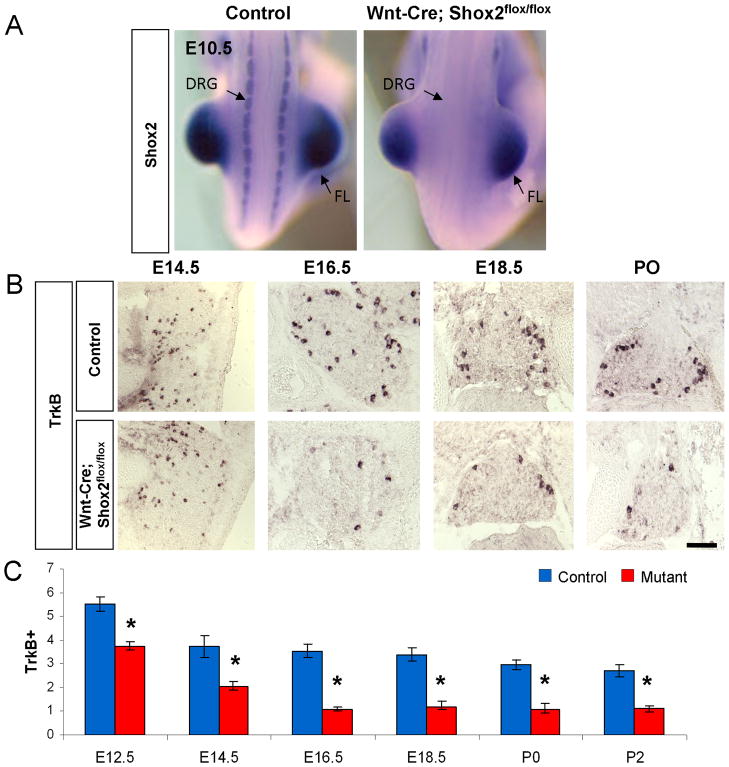
To examine the function of Shox2 in DRG neuron development, we used Wnt1-Cre (Danielian et al., 1998) to conditionally delete the Shox2 gene in all neural crest derived cells including DRG neurons. We crossed Wnt1-Cre mice with Shox2flox/flox mice (Cobb et al., 2006; Danielian et al., 1998) to obtain conditional mutant Wnt1-Cre; Shox2flox/flox (as well as control Wnt1-Cre; Shox2flox/+) embryos and neonates. Figure 2A shows the absence of Shox2 expression in DRG from Shox2-mutant embryo at E10.5. In our hands, all Shox2 mutant mice die within two days after birth due to an anterior cleft palate defect (data not shown), which limited our characterization of the role of Shox2 to embryonic and neonatal stages.
Figure 2. Reductions in the number of TrkB-expressing DRG neurons in Wnt1-Cre; Shox2flox/flox embryos.
A. In situ hybridization reveals the absence of Shox2 expression in DRGs of Wnt1-Cre; Shox2flox/flox mice at E10.5. B. Representative images of TrkB expression in control and Shox2-deleted DRG at E14.5, E16.5, E18.5 and P0. C. Quantifications of the numbers of TrkB-expressing DRG neurons per unit area in control and Wnt1-Cre; Shox2flox/flox embryos. *p<0.001. Error bars represent ±SEM. Scale bar = 100μm.
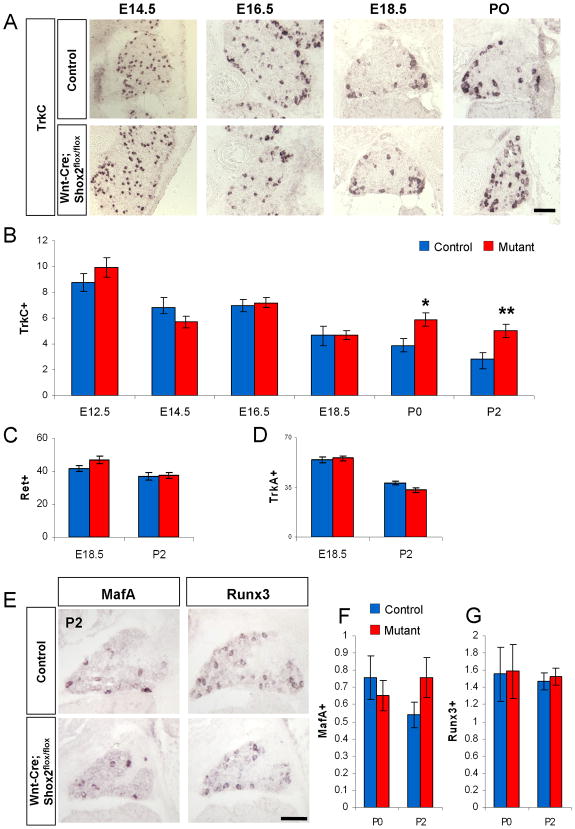
Using in situ hybridization, we examined the expression of Trks and Ret receptors in developing DRG at different stages in the mutant and control embryos. There is no observable difference in the number of TrkA or Ret positive cells at all time points examined, suggesting that Shox2 does not play a major role in the development of nociceptive lineage (Figure 3C–D). In contrast, we observed a significant decrease in the number of TrkB-expressing cells in the Shox2-deleted DRG compared with controls at all time points examined (Figure 2B–C). On average, there is a 60~65% loss of TrkB-expressing DRG neurons at stages after E16.5 (Figure 2C), which is consistent with the fact that 65% TrkB-neurons express Shox2. Finally, the proprioceptive lineage marker TrkC showed a mild increase in the Shox2-deleted DRG at perinatal stages starting after E18.5 (Figure 3A–B). The detailed method used for quantifying the numbers of different types of DRG neurons in these and subsequent results is described in Materials and Method.
Figure 3. Increases in the number of TrkC-expressing DRG neurons in Wnt1-Cre; Shox2flox/flox mice at perinatal stages.
A. Representative images of TrkC expression in control and Shox2-deleted DRG at E14.5, E16.5, E18.5 and P0. B. Quantifications of the numbers of TrkC-expressing DRG neurons per unit area in control and Wnt1-Cre; Shox2flox/flox embryos at 6 different stages. Note that there is a increase in the number of TrkC expressing neurons in the mutant at P0 (*p<0.005) and P2 (**p<0.001). C and D. Quantification of the number of Ret and TrkA expressing neurons in control and Wnt1-Cre; Shox2flox/flox mice at E18.5 and P2. Error bars represent ±SEM. Scale bar = 100μm. E. Representative images of MafA and Runx3 expression in control and Shox2-deleted DRGs at P2. F and G. Quantifications show no significant differences in the numbers of cells expressing MafA or Runx3 per unit area between the control and Shox2-deleted DRG at P0 and P2.
Previous studies have shown that the transcription factors MafA and Runx3 are involved in the development of the Ret-positive mechanosensory neurons and TrkC-positive proprioceptive neurons, respectively (Bourane et al., 2009; Inoue et al., 2007; Kramer et al., 2006). We used in situ hybridization to examine the expression of both these transcription factors in mutant and control mice. We found that there was no difference in the number of DRG neurons expressing either MafA or Runx3 in the Shox2-deleted verses control DRG at P0 or P2 (Figure3E–G). The result suggests that Shox2 do not regulate MafA or Runx3 expression, and by extension, probably do not play a major role in the development/specification of Ret-positive rapid adapting mechanosensory neurons or TrkC-expressing proprioceptive neurons.
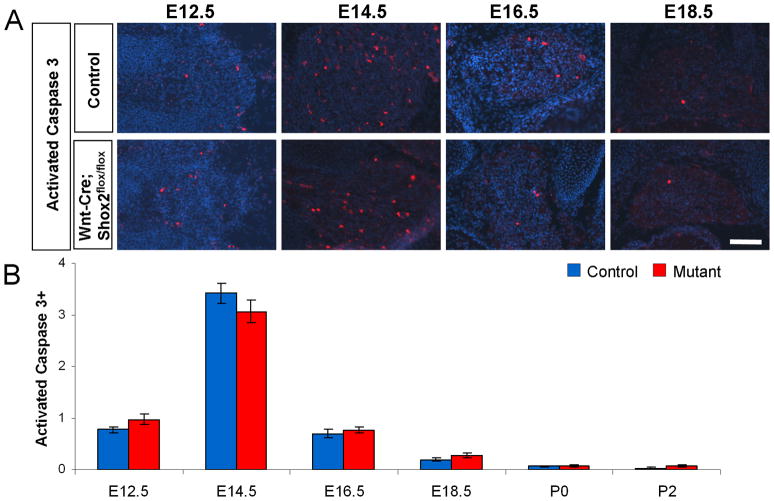
Loss of TrkB-expressing DRG neurons in Wnt1-Cre; Shox2flox/flox mouse is not caused by elevated apoptosis
We next investigated whether apoptosis could account for the observed loss of TrkB-expressing neurons in Wnt1-Cre; Shox2flox/flox DRG. We used anti-activated caspase-3 antibody to detect cell death, and found no statistically significant difference in the number of apoptotic cells in Shox2-deleted versus control DRG at all time points examined (Figure 4A–B). Note that both Shox2-deficient and control DRG show increased numbers of caspase-3 positive cells at E14.5 (Figure 4B), a time point of natural occurring cell death of developing sensory neurons as shown previously (Raff et al., 1993; White et al., 1998). Thus, the loss of TrkB-expression in Shox2 mutant mice is not likely due to apoptosis of sensory neurons.
Figure 4. Apoptosis is normal in Wnt1-Cre; Shox2flox/flox DRG.
A. Representative images of immunostaining with anti-activated-caspase-3 in control and Shox2-deleted DRG at E12.5, E14.5, E16.5, and E18.5. B. Quantifications show no significant differences in the amount of apoptotic cells between control and Shox2-deleted DRG. Note that at E14.5, a time point of natural occurring cell death, there is an increase in activated-caspsase-3 positive cells in both control and mutant DRG. Error bars represent ±SEM. Scale bar = 100μm.
TrkC co-expression in subsets of TrkB- or Ret-positive sensory neurons in Wnt1-Cre; Shox2flox/flox mouse
It is known that during early DRG neurogenesis, the transient population of TrkC/TrkB double positive progenitor neurons later differentiate into TrkB-single positive or Ret-single positive mechanosensory neurons, or TrkC-single positive proprioceptive neurons (Kramer et al., 2006; Marmigere and Ernfors, 2007). However, there appears to be a small percent of sensory neurons that maintain co-expression of TrkB/TrkC or Ret/TrkC. We thus examined the co-expression of TrkC in the remaining TrkB-, as well as in Ret-expressing DRG neurons in Shox2 mutant mice.
We first performed fluorescent two-color in situ hybridization to detect TrkB and TrkC mRNA simultaneously. In control DRG at E14.5, about 33% of TrkB positive neurons also express TrkC. By E16.5, less than 10% of TrkB-cells still express TrkC in wild type DRG. This number is further reduced during postnatal development (Figure 5A). In Wnt1-Cre; Shox2flox/flox DRG, there is an apparent increase in the relative percentage of TrkB/TrkC double positive cells. However, when we quantified the actual average numbers of TrkB/TrkC double positive DRG neurons per unit area, there is no statistically significant difference between control and Shox2-mutant DRG (Figure 5B). This result suggests that normally in wildtype mouse, TrkB/TrkC double positive mechanosensory neurons belong to the 35% TrkB-positive but Shox2-negative populations, and thus their number is unaffected by Shox2-deletion.
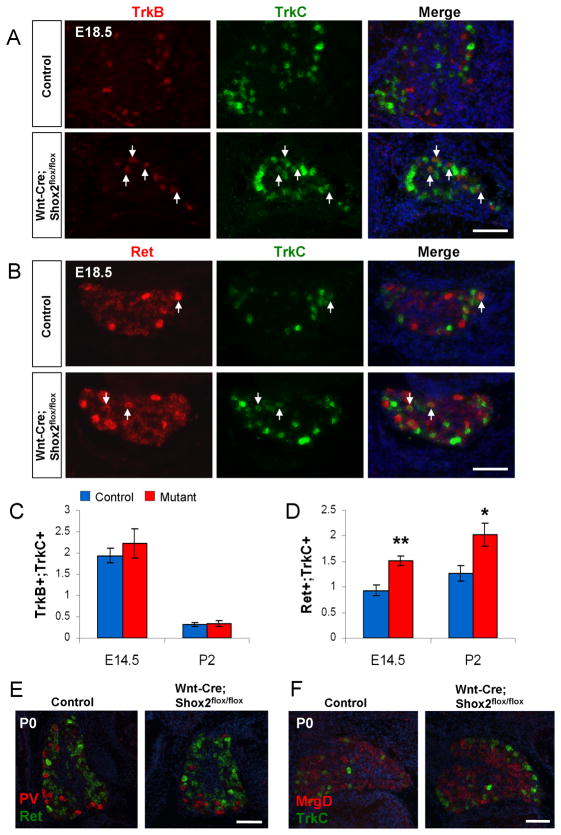
Figure 5. Increased co-expression of Ret and TrkC in DRG neurons in Wnt1-Cre; Shox2flox/flox mice.
A. Representative results of two-color in situ hybridization using TrkB (red) and TrkC (green) probes at E18.5. Arrows indicate neurons in the DRG that are positive for both receptors. B. Representative results of two-color in situ hybridization using Ret (red) and TrkC (green) probes at E18.5. Arrows indicate cell bodies in the DRG that are positive for both receptors. C. Quantification of the average number (per unit area) of TrkB/TrkC double positive neurons revealed no significant difference between the control and Shox2-deleted mice. D. Quantification of the average number (per unit area) of Ret/TrkC double positive neurons showed a significant increase in the Shox2-deleted verses control DRG. E. Representative images of two color in situ hybridization results with Parvalbumin (red) and Ret (green) probes at P0. F. Representative images of two color in situ hybridization results with MrgD (red) and TrkC (green) probes at P0. *p<0.01 and **p<0.001. Scale bar = 100μm.
Since Shox2 is also expressed in a subset of Ret-positive neurons (Figure 1B), we also examined co-expression of TrkC in Ret-expressing sensory neurons. Again, using two-color in situ hybridization, we found that there are a small percentage of Ret-positive cells also expressing TrkC at perinatal stages in both control and Shox2-mutant DRG. Interestingly, the average number of Ret/TrkC double positive neurons per unit area is increased in the mutant (Figure 5C–D). This result is consistent with the observed increase in the total number of TrkC-expressing DRG neurons, and suggests that normally, the function of Shox2 in Ret-positive neurons may be to suppress TrkC expression.
To examine whether any of the Ret/TrkC double positive cells in Shox2-deleted DRG differentiate toward a proprioceptive neuron fate, we carried out two-color in situ hybridization to detect Ret and Parvalbumin (PV) simultaneously. Parvalbumin is a known marker for TrkC expressing proprioceptive sensory neurons (Arber et al., 2000). Ret/PV double positive cells are rarely seen in either control or Shox2-mutant DRG, and there is no statistically significant difference in the total number of PV positive neurons between control and mutant mice (Figure 5E and data not shown). This result suggests that the increased number of Ret/TrkC double positive neurons in Shox2-mutant DRG do not appear to be proprioceptive neurons. To determine whether any of the Ret/TrkC double-positive neurons belong to the late-born Ret-positive nociceptive neurons, we performed two-color in situ using MrgD and TrkC probes. MrgD is expressed exclusively in Ret/Runx1-expressing non-peptidergic nociceptive neurons (Liu et al., 2008). We did not find any MrgD/TrkC double positive neurons in either control or Shox2-mutant mice (Figure 5F). Thus, by exclusion, the increased Ret/TrkC double positive neurons in Shox2-mutant are mechanosensory neurons.
Taken together, Shox2-deletion resulted in a significant loss of TrkB expression and a mild increase of TrkC co-expression in small number of Ret-positive mechanosensory neurons. These data suggest that Shox2 is essential for the proper expression of neurotrophic receptors during the differentiation of mechanosensory neurons.
Developmental time-dependent requirement of Shox2 for proper TrkB expression in DRG neurons
Previous studies have found time-dependent roles of certain transcription factors (ETS genes, Islet1) in DRG neuron development (Hippenmeyer et al., 2005; Sun et al., 2008). We therefore wanted to determine the time window when Shox2 is required for the development of a subset of TrkB-expressing sensory neurons. We used AvilCre/+ (Zhou et al., 2010) to delete Shox2 gene at later stages by generating AvilCre/+; Shox2flox/flox mice. Advillin is a gene whose expression is largely restricted to peripheral sensory neurons. Advillin is weakly expressed in a few DRG neurons at E12.5 and reaches peak expression at E16.5 (da Silva et al., 2011; Hasegawa et al., 2007; Zhou et al., 2010). AvilCre/+ mediated deletion of Shox2 is completed at E18.5 (Figure 6A).
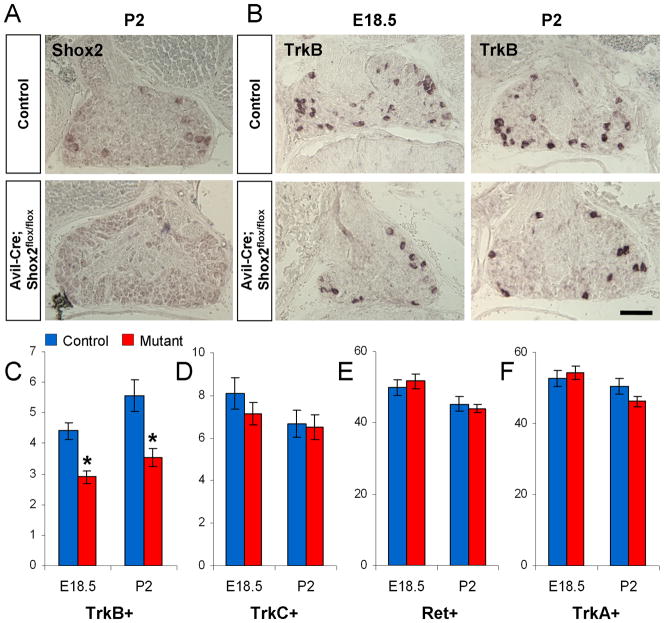
Figure 6. Mild reduction in the number of TrkB-expressing cells in AvilCre/+; Shox2flox/flox mice.
A. In situ hybridization confirms the loss of Shox2 expression in the DRG from AvilCre/+; Shox2flox/flox mice. B. Representative images of TrkB expression in control and AvilCre/+; Shox2flox/flox mice at E18.5 and P2. C–F. Quantifications of the numbers of TrkB (C), TrkC (D), Ret (E), and TrkA (F) expressing DRG neurons per unit area in control and AvilCre/+; Shox2flox/flox mice at E18.5 and P2. *p<0.001. Error bars represent ±SEM. Scale bar = 100μm.
The AvilCre/+; Shox2flox/flox mice are viable, fertile and appear normal. Using in situ hybridization, we found that the number of TrkB positive cells is only moderately decreased in these mutants compared to the controls (Figure 6B–C). Furthermore, there is no difference in the numbers of TrkC, TrkA or Ret positive neurons between AvilCre/+; Shox2flox/flox and the control DRG (Figure 6D–F). Thus, deleting Shox2 at stages after E12.5 resulted in a milder loss of TrkB expression and no effect on TrkC expression, suggesting Shox2 is required primarily at the very early stages of mechanosensory neuron differentiation to ensure normal Trk receptor expression.
Effects of induced constitutive expression of Shox2 in all neural crest derived cells
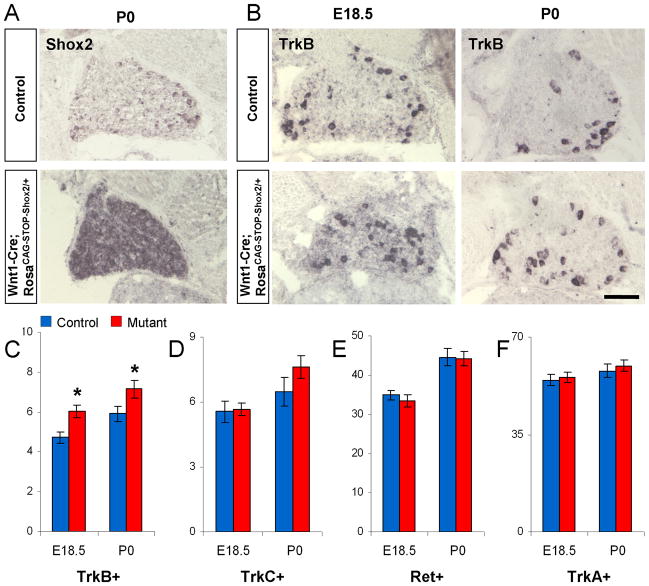
To gain further insight into the functions of Shox2, we asked whether over-expressing Shox2 in all DRG neurons would have a dominant effect on Trk receptor expression. To achieve this, we created a knock-in mouse that allows Cre-dependent over-expression of Shox2. Briefly, a CAG promoter followed by loxP-STOP-loxP cassette, followed by Shox2 cDNA and polyA was inserted into Rosa26 locus (RosaCAG-STOP-Shox2). The knock-in mice were crossed to Wnt1-Cre to obtain Wnt1-Cre; RosaCAG-STOP-Shox2/+ mice. In situ hybridization confirmed the over-expression of Shox2 mRNA in all DRG cells (Figure 7A). Wnt1-Cre; RosaCAG-STOP-Shox2/+ mice die immediately at birth, due to a completely cleft palate (data not shown). We examined the expression of Trks and Ret in these mice at E18.5 and P0 stages. Upon quantification, we found a 20% increase in the number of TrkB positive neurons in DRG from Shox2-overexpression embryos (Figure 7B–C). However, no apparent changes in TrkA, TrkC and Ret expression were detected in these mice compared to the controls (Figure 7D–F). Thus, although Shox2 is necessary for inducing and/or maintaining TrkB expression in subsets of mechanosensory neurons, it is not sufficient to induce TrkB or suppress TrkC expression in all DRG neurons.
Figure 7. Over-expression of Shox2 in all sensory neurons results in a mild increase in the number of TrkB-positive cell.
A. Representative in situ hybridization images confirming the overexpression of Shox2 in all DRG neurons in Wnt1-Cre; RosaCAG-STOP-Shox2 mice. B. Representative images of TrkB in situ hybridization results in control and Shox2 overexpression mice. C–F. Quantifications of the numbers of TrkB (C), TrkC (D), Ret (E), and TrkA (F) expressing DRG neurons per unit area in control and Shox2 overexpression mice at E18.5 and P0. *p<0.05. Error bars represent ±SEM. Scale bar = 100μm.
Defects in mechanosensory neuron central innervations in Shox2-deficient mice
Finally, we examined the consequences of loss of Shox2 on the peripheral and central axonal projections of mechanosensory DRG neurons. To visualize the axonal projections, we utilized the AvilPLAP/+ mice in which human placenta alkaline phosphatase (PLAP) is inserted into the Advillin locus (Hasegawa et al., 2007). We crossed Wnt1-Cre; Shox2flox/+ males to AvilPLAP/+; Shox2flox/flox females to generate AvilPLAP/+; Wnt1 -Cre; Shox2flox/flox mutant mice and their littermate controls. Since no mutant mice survive past P2, we examined axonal projection at E18.5, P0 and P2. At these stages, the peripheral axons have reached their targets, but have not yet fully differentiated into specialized sensory endings (Albuerne et al., 2000; Hasegawa et al., 2007), thereby preventing us from definitively determining the exact morphological subtypes of neurons that are affected by Shox2 deletion. Using PLAP staining and anti-PGP9.5 staining, we did not detect any apparent differences in the general peripheral sensory projections into the hairy or the glabrous skin (data not shown).
However, PLAP staining on spinal cord sections from control or AvilPLAP/+; Wnt1 -Cre; Shox2flox/flox mutant mice revealed that the Shox2-deleted DRG neurons showed reduced central axonal innervation to layers III/IV of the spinal cord compared to the control (Figure 8). Note that layer III/IV stained strongly for PLAP in the control (arrow in Figure 8A), but the corresponding region in the Wnt1-Cre; Shox2flox/flox mutant spinal cord stained much weaker (Figure 8A). On average, there is a 10% reduction in the PLAP-staining intensity in Shox2-mutant (p<0.001). Since layers III/IV receive inputs from mechanosensory neurons, including TrkB and Ret-expressing touch neurons, this observation suggests that Shox2 deficiency causes central innervation defects in subsets of mechanosensory neurons. Note that the reduced PLAP staining in layer III/IV was not due to changes in the expression of Advillin locus as PLAP staining in the DRG cell bodies and peripheral axons were equally intense, and in situ hybridization showed similar level of Advillin expression in both control and Shox2-deleted DRG (data not shown).
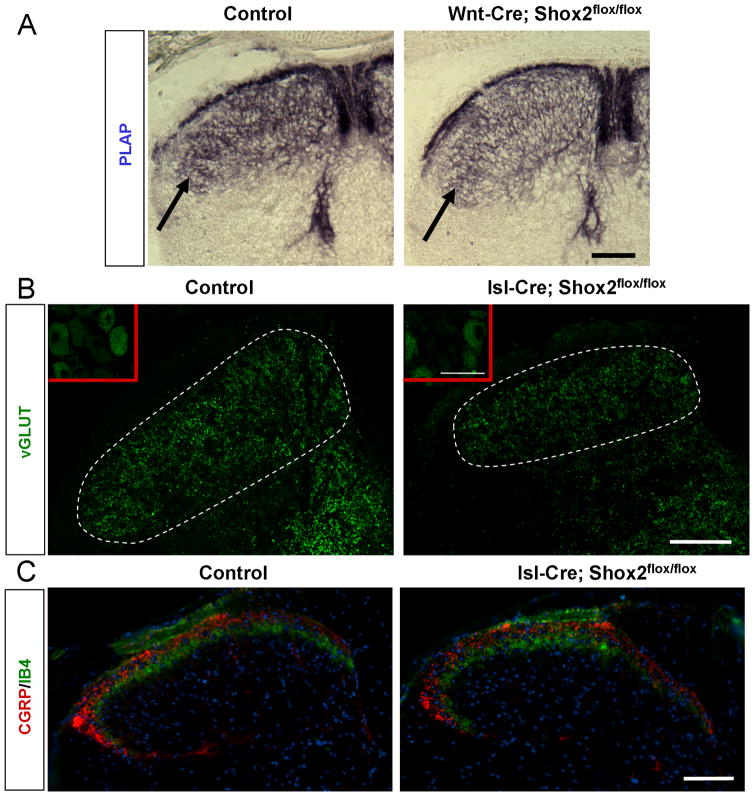
Figure 8. Reduced mechanosensory central innervation in the spinal cord in Shox2-deleted mice.
A. Representative images of sensory afferent projections in the spinal cord from control (Wnt1-Cre; Shox2flox/+;AvilPLAP/+) and Shox2-deleted mice (Wnt1-Cre; Shox2flox/flox;AvilPLAP/+) as revealed by PLAP staining at P0. Arrows point to a densely stained band in layer III/IV in control that is only moderately stained in the mutant. B. Representative images of immunofluorescence staining with anti-vGluT1 in the spinal cord of control (Isl1-Cre; Shox2flox/+) and Shox2-deleted (Isl1-Cre; Shox2flox/flox) mice. Inset shows the anti v-GluT1 immunofluorescence signal in the DRG of control and Shox2-deleted mice. C. Representative images of immunofluorescence staining with anti-CGRP (red) and anti-IB4 (green) in the spinal cord of control and Shox2-deleted mice. Blue is DAPI. Scale bar in A and C is 100μm. Scale bar in B is 50μm.
To confirm the central axon innervation defects with an independent method, we also used Isl1cre/+ (Srinivas et al., 2001) to conditionally delete Shox2. Islet1 (Isl1) is expressed in all sensory neurons beginning at E10, thus in these mice Shox2 is deleted at early stages of DRG neuron differentiation. In Isl1cre/+; Shox2flox/flox mice, we observed 53% reduction in the number of TrkB expressing cells, only slightly less than what we observed in Wnt1 -Cre; Shox2flox/flox mice (p<0.001, data not shown). We used anti-vGlut1 staining to specifically visualize mechanosensory afferent termini (whereas AvilPLAP/+ labels all axons including nociceptive afferents). The average vGluT1 staining intensity in layer III/IV showed an 11% reduction in Isl1cre/+; Shox2flox/flox dorsal spinal cord compared to that in controls (Figure 8B, p<0.002). As a control, the vGLUT staining signals in DRG neuronal cell bodies were indistinguishable between control and Shox2-mutant mouse (insets in Figure 8B). In addition, the average area covered by vGluT1 positive mechanosensory axons in dorsal horn spinal cord is also reduced by about 20% in Isl1cre/+; Shox2flox/flox mice (Figure 8B, p<0.001). Not surprisingly, nociceptive innervation to the dorsal horn as revealed by anti-CGRP and IB4 staining (to visualize peptidergic and nonpeptidergic nociceptive central axonal projections, respectively) was unchanged in Shox2-mutant mice (Figure 8C). Taken together, our study uncovered an important requirement for the transcription factor Shox2 for the proper development, differentiation and central innervation of subset of TrkB-expressing mouse mechanosensory neurons.
Discussion
In the mammalian peripheral somatosensory system, mechanosensory and proprioceptive lineages arise from the same progenitor populations, through the first wave of neurogenesis from the precursor NCCs that migrate into the site of the future DRG at E9 (Fode et al., 1998; Ma et al., 1999). The initially TrkC/TrkB double positive progenitor neurons eventually differentiate into TrkC single positive proprioceptive neurons, as well as TrkB or Ret single positive touch sensory neurons, although a very small number of mechanosensory neurons are TrkB/TrkC or Ret/TrkC double positive. Previously, the molecular mechanisms regulating the specification of TrkB-expressing mechanosensory neuron lineage are unknown. In this study, we discovered that Shox2 is an essential, although not sufficient, component required for the proper development of a subpopulation of TrkB positive DRG neurons.
Wnt1-Cre mediated deletion of Shox2 in NCCs result in a 60–65% reduction in the number of TrkB-expressing DRG neurons at stages after E16.5. Shox2-deletion also caused a small increased in TrkC/Ret double positive neurons at later stages. These findings suggest that Shox2 is important for ensuring TrkB expression and may contribute to TrkC repression in Ret-expressing touch sensory neurons, although at present we do not yet know whether such effects of Shox2 is direct or indirect.
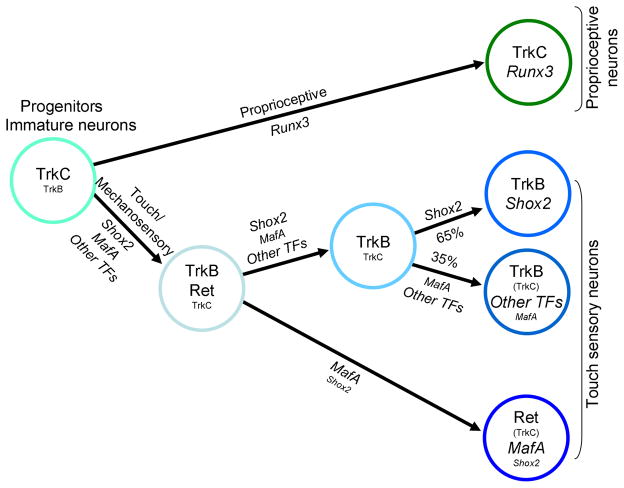
Later deletion of Shox2 using AvilCre/+ resulted in only a moderate decrease in TrkB positive neurons than that observed in Wnt1-Cre mediated deletion mice, suggesting Shox2 is required in progenitor and early born neurons to promote their differentiation into TrkB-expressing touch neurons. The onset of Advillin expression is at E12.5 and reaches a maximum at E16.5. It is likely that only those neurons that express Cre at early stages (E12.5) are affected and lose TrkB expression, whereas those that express Cre after E12.5 are not affected by Shox2 deletion. Perhaps that once the stable high-level expression of TrkB is established, Shox2 is no longer needed for the maintenance of TrkB expression. In our gain-of-function studies, overexpression of Shox2 in all NCC progenitor cells only mildly increased the number of TrkB-positive cells, but had no effects on other Trks or Ret receptor expressions, including TrkC. Taken together, Shox2 is necessary for inducing or maintaining TrkB expression in a subpopulation of mechanosensory neurons, but itself alone is not sufficient to induce TrkB expression in non-mechanosensory neurons. A model summarizing previous and current finding related to proprioceptive and mechanosensory neuron specification is shown in Figure 9.
Figure 9. A model for the development and diversification of propriocptive and touch sensory neurons.
Schematic model shows the transcription factors involved in the progressive specification of TrkB/TrkC double positive progenitor/immature neurons into proprioceptive and different types of touch sensory neurons.
At present, we do not know the downstream targets of Shox2, nor do we know whether Shox2 only regulates Trk receptor expression or whether it also regulates other genes involved in other aspects of the touch/mechanosensory neuronal development. In other sensory lineages, the transcription factors Runx3 and Runx1 control the whole gene expression programs relevant to proprioceptive or nociceptive sensory neurons development and differentiation, respectively (Chen et al., 2006; Inoue et al., 2008; Kramer et al., 2006). On the other hand, the transcription factor Klf7 is required only for TrkA gene expression by binding to an enhancer element in the TrkA promoter. The loss of Klf7 leads to increased apoptosis of nociceptive sensory neurons. However, Klf7 did not appear to regulate other aspects of the differentiation program of TrkA-positive neurons (Lei et al., 2005). Future work is needed to determine the transcription targets of Shox2.
In Shox2-deficient mice, the central afferent innervations from mechanosensory neurons to layers III/IV in the spinal cord are reduced. Again, this could be a direct consequence of failed differentiation of a subset of TrkB-expressing touch neurons, or a secondary effect due to the loss of TrkB expression. Unlike the wealth of marker genes known for proprioceptive or nociceptive neurons, very limited molecular markers are known to specifically label TrkB-expressing mechanosensory neurons. We, therefore, could not examine other molecular aspects of mechanosensory neuron development and differentiation in Shox2 mutant mice. Nonetheless, Shox2 is the first transcription factor discovered that affects TrkB expression in mechanosensory DRG neurons.
Acknowledgments
We thank members of the Wang laboratory for helpful discussions, suggestions and technical assistance. We thank Dr. Scott Soderling and Terry Lechler for critical reading of the manuscript. We thank the Duke Transgenic Mouse Facility for making the RosaCAG-STOP-Shox2/+ mice. Dr. Jeh-Ping Liu for providing the Isl1-Cre mice. This work is supported by NIH grants from NIDCR and McKnight Scholar for Neuroscience Award to F.W, as well as US-Israel Binational Science Foundation to A. Y and F.W. In addition, J.C. is supported as an Alberta Heritage Foundation for Medical Research (AHFMR) Scholar and by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes of Health Research (CIHR) Grant # MOP-93562.
Footnotes
Conflicts of Interest: None
References
- Albuerne M, De Lavallina J, Esteban I, Naves FJ, Silos-Santiago I, Vega JA. Development of Meissner-like and Pacinian sensory corpuscles in the mouse demonstrated with specific markers for corpuscular constituents. Anat Rec. 2000;258:235–242. doi: 10.1002/(SICI)1097-0185(20000301)258:3<235::AID-AR2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Binder G. Short Stature due to SHOX Deficiency: Genotype, Phenotype, and Therapy. Horm Res Paediatr. 2011;75:81–89. doi: 10.1159/000324105. [DOI] [PubMed] [Google Scholar]
- Blaschke RJ, Hahurij ND, Kuijper S, Just S, Wisse LJ, Deissler K, Maxelon T, Anastassiadis K, Spitzer J, Hardt SE, et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation. 2007;115:1830–1838. doi: 10.1161/CIRCULATIONAHA.106.637819. [DOI] [PubMed] [Google Scholar]
- Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron. 2009;64:857–870. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Cobb J, Dierich A, Huss-Garcia Y, Duboule D. A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc Natl Acad Sci U S A. 2006;103:4511–4515. doi: 10.1073/pnas.0510544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva S, Hasegawa H, Scott A, Zhou X, Wagner AK, Han BX, Wang F. Proper formation of whisker barrelettes requires periphery-derived Smad4-dependent TGF-{beta} signaling. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1014411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva S, Wang F. Retrograde neural circuit specification by target-derived neurotrophins and growth factors. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Germana GP, Monjil DF, Silos-Santiago I, de Carlos F, Germana G, Cobo J, Vega JA. Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain Res. 2004;1002:120–128. doi: 10.1016/j.brainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci. 2007;27:14404–14414. doi: 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Wang F. Visualizing mechanosensory endings of TrkC-expressing neurons in HS3ST-2-hPLAP mice. J Comp Neurol. 2008;511:543–556. doi: 10.1002/cne.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge LK, Klassen MP, Han BX, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, Wang F. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55:572–586. doi: 10.1016/j.neuron.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ito K, Osato M, Lee B, Bae SC, Ito Y. The transcription factor Runx3 represses the neurotrophin receptor TrkB during lineage commitment of dorsal root ganglion neurons. J Biol Chem. 2007;282:24175–24184. doi: 10.1074/jbc.M703746200. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shiga T, Ito Y. Runx transcription factors in neuronal development. Neural Dev. 2008;3:20. doi: 10.1186/1749-8104-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lei L, Laub F, Lush M, Romero M, Zhou J, Luikart B, Klesse L, Ramirez F, Parada LF. The zinc finger transcription factor Klf7 is required for TrkA gene expression and development of nociceptive sensory neurons. Genes Dev. 2005;19:1354–1364. doi: 10.1101/gad.1227705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon D, Brenner O, Negreanu V, Bettoun D, Woolf E, Eilam R, Lotem J, Gat U, Otto F, Speck N, et al. Spatial and temporal expression pattern of Runx3 (Aml2) and Runx1 (Aml1) indicates non-redundant functions during mouse embryogenesis. Mech Dev. 2001;109:413–417. doi: 10.1016/s0925-4773(01)00537-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci. 2008;28:125–132. doi: 10.1523/JNEUROSCI.4472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini A, Rappold G, Schneider KU. SHOX at a glance: from gene to protein. Arch Physiol Biochem. 2007;113:116–123. doi: 10.1080/13813450701531201. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Garcia-Suarez O, Germana A, Diaz-Esnal B, de Carlos F, Silos-Santiago I, del Valle ME, Cobo J, Vega JA. Characterization of sensory deficits in TrkB knockout mice. Neurosci Lett. 2008;433:43–47. doi: 10.1016/j.neulet.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Sedy J, Tseng S, Walro JM, Grim M, Kucera J. ETS transcription factor ER81 is required for the Pacinian corpuscle development. Dev Dyn. 2006;235:1081–1089. doi: 10.1002/dvdy.20710. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ichikawa H, Nakagawa H, Kiyomiya K, Matsuo S. Effect of BDNF depletion on the formation of Ruffini endings in vibrissa follicles and the survival of their mechanoreceptive neurons in trigeminal ganglion. Brain Res. 2007;1154:95–104. doi: 10.1016/j.brainres.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat Neurosci. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Dev Biol. 2003;253:258–263. doi: 10.1016/s0012-1606(02)00015-5. [DOI] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Gu S, Alappat S, Song Y, Yan M, Zhang X, Zhang G, Jiang Y, Zhang Z, Zhang Y, et al. Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development. 2005;132:4397–4406. doi: 10.1242/dev.02013. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, He Y, Wang F. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci U S A. 2010;107:9424–9429. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]