Figure 3.
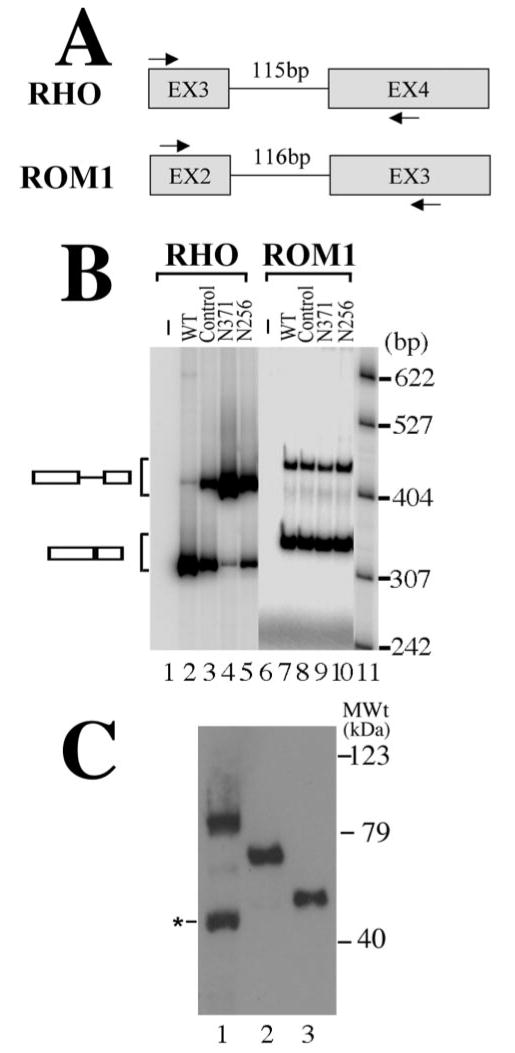
Mutant PRPF31 expression inhibits the splicing of RHO intron 3 but not ROM1 intron 2. A, A diagram is shown for RHO and ROM1 minigenes. The arrows mark the positions of specific primers used in RT-PCR assay to detect the corresponding splicing products. B, Effects of PRPF31 mutant proteins on the pre-mRNA splicing of RHO intron 3 and ROM1 intron 2 in transfected cells. After cotransfection of plasmids expressing wild-type (lanes 2 and 7), GFP vector control (lanes 3 and 8), or mutant [N371 (lanes 4 and 9) or N256 (lanes 5 and 10)] proteins together with either the RHO intron 3 minigene or the ROM1 intron 2 minigene into HEK cells, the corresponding pre-mRNA and splicing products are detected using RT-PCR with specific primers in the corresponding regions as shown in A. Lanes 1 and 6 contain the RT-PCR products prepared from cells transfected with the empty vector not containing either RHO or ROM1 minigene inserts. Lanes 2–5 (RHO minigene) and lanes 7–10 (ROM1 minigene) contain the RT-PCR products from cells transfected with the corresponding minigenes. DNA size markers are in lane 11, with sizes indicated on the right. C, The expression levels of wild-type and mutant PRPF31 proteins are equivalent in transfected HEK cells. The expression plasmids that were used for transfecting retinal cells (Figs. 4, 5) were transfected into HEK cells, and the GFP fusion proteins were detected by Western blotting using an anti-GFP antibody. Lane 1, Wild type; lane 2, N371; lane 3, N256. A degradation band of wild-type PRPF31–GFP fusion protein (marked by the asterisk) was sometimes detectable. MWt, Molecular weight.
