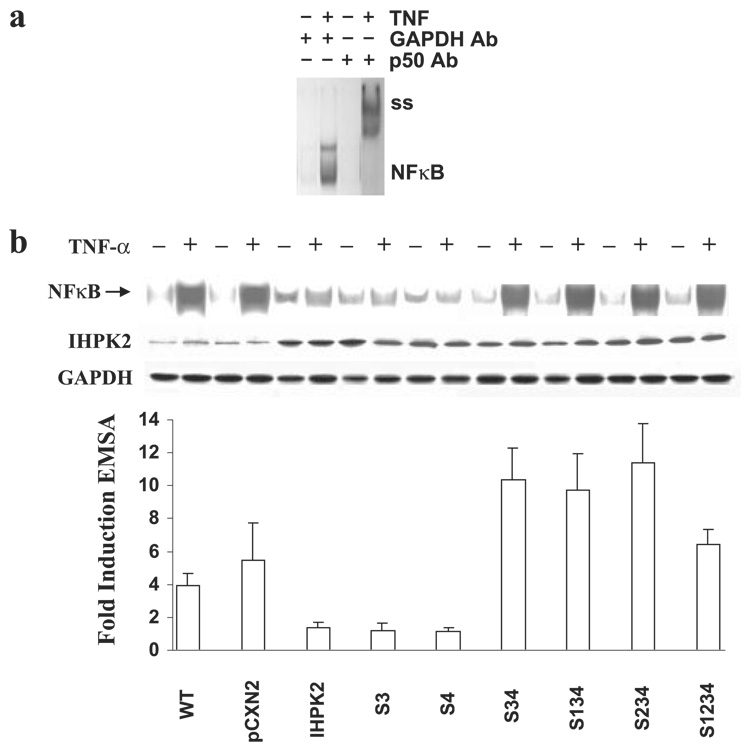
FIGURE 5. Effect of IHPK2 mutation upon NF-κB DNA binding activity.
a, wild type untransfected NIH-OVCAR-3 cells were treated with TNF-α, and EMSA was performed. Lysates were incubated with anti-GAPDH or anti-NF-κB (p50) prior to EMSA to demonstrate supershift (ss). b, cells transfected with IHPK2 S3&4 mutations (S347A and S359A) displayed enhanced NF-κB DNA binding activity induced by TNF-α. Cells transfected with wild type IHPK2 or IHPK2 mutants that lacked S3&4 mutations had suppressed NF-κB DNA binding activity. Cells were stimulated with TNF-α (20 ng/ml) for 15 min. Expression of IHPK2 transgene is indicated, and GAPDH served as loading control. EMSA band intensities were quantified with a Phosphorimager, and -fold induction was calculated. Cells expressing IHPK2 mutants S3&4, S1&3&4, S2&3&4, and S1&2&3&4 were 7- to 11-fold more effective at activation of NF-κB compared with cells transfected with wild type IHPK2, whereas cells expressing IHPK2 and single mutants had <2-fold induction. Data are expressed as mean ± S.E. of three separate experiments.