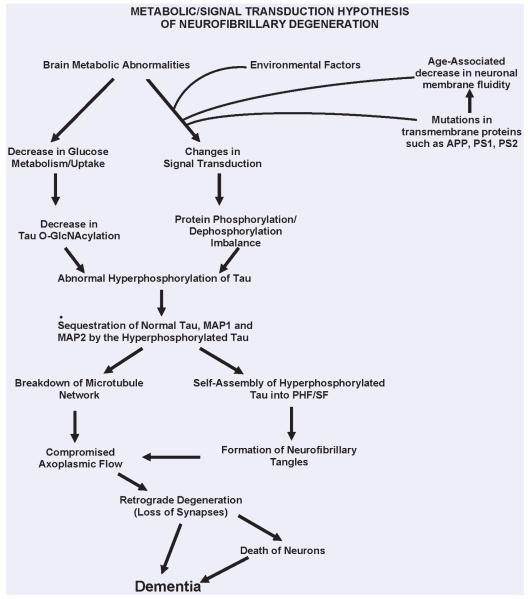
Fig. 1.
A schematic showing different major steps of the ‘Metabolic/Signal Transduction Hypothesis’. Alzheimer disease (AD) and other tauopathies require a genetic predisposition and are triggered by a variety of environmental factors, affecting one or more specific signal transduction pathways which result in a protein phosphorylation/dephosphorylation imbalance and the abnormal hyperphosphorylation of tau that leads to neurofibrillary degeneration and dementia. In AD, the protein phosphorylation/dephosphorylation imbalance in the affected neurons is generated at least in part by a decrease in the activities of tau phosphatases, that is PP-2A and PP-1; the activities of tau kinases, such as cdk5, GSK-3, CaM kinase II and PKA might also be increased in the affected neurons. This protein phosphorylation/dephosphorylation imbalance probably involves an alteration of a specific signal transduction pathway(s) produced by an increase in the levels of an extracellular signal, for example, FGF2 or an alteration in the molecular topology of the neuronal cell membrane or both. With age, the molecular topology of the cell membranes is altered due to a decrease in membrane fluidity. The mutations in transmembrane proteins, such as β-APP, PS1 and PS2, increase the vulnerability of the cell membrane to alteration in pathological signal transduction. The increased risk for AD in the carriers of APOE4 allele as opposed to APOE2 or APOE3 alleles might also involve alteration of signal transduction through the interaction of APOE4 with the neuronal cell membrane. Any mutation or posttranslational modification of tau that will make it a better substrate for abnormal hyperphosphorylation will also increase the risk for the disease. High cholesterol might be involved in decreasing membrane fluidity. Decreased glucose metabolism/uptake might lead to the abnormal hyperphosphorylation of tau through a decrease in its O-GlcNAcylation. (Reproduced with permission from Iqbal and Grundke-Iqbal, Acta Neuropathologica, 2005, 109:25-31).