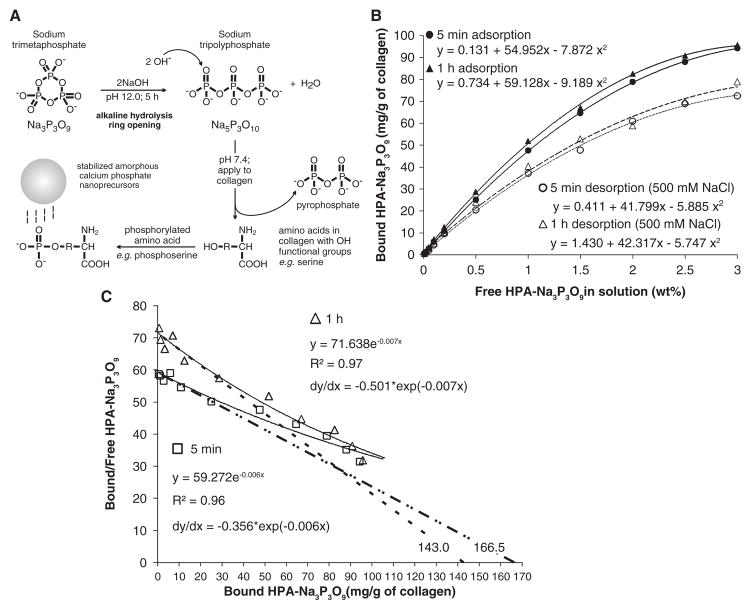
Figure 1.
Adsorption and desorption characteristics of sodium trimetaphosphate. (A) Sodium trimetaphosphate (Na3P3O9) was used for introducing a matrix phosphoprotein analog to collagen. Ring opening was achieved by alkaline hydrolysis at pH 12 for 5 hrs to produce sodium tripolyphosphate (Na5P3O10). The pH of Na5P3O10 was adjusted to 7.4 to retain its phosphorylation potential. The phosphorylated amino acids on the collagen molecules function as templates for attracting polyacrylic-acid-stabilized amorphous calcium phosphate nanoprecursors. (B) Adsorption and desorption isotherms of different ligand concentrations (hydrolyzed and pH-adjusted Na3P3O9, hereafter referred to as HAP-Na3P3O9) to demineralized collagen matrices for 5 min and 1 hr at 37°C. Each point represents the mean of triplicate assays. Twelve different HAP-Na3P3O9 concentrations (wt%) were used: 0, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1.0, 1.5, 2.0, 2.5, and 3. (C) Corresponding Scatchard plot transformed from the 5-minute (open squares) and one-hour (open triangles) adsorption data in (A) yielded nonlinear plots (solid lines) that are suggestive of several populations of binding sites in the collagen matrix, with different ligand affinities. The tangent (dotted lines) of each regressive curve at the point of intersection of the curve with the Y-axis was extrapolated to determine the optimal free ligand concentration for irreversible binding.