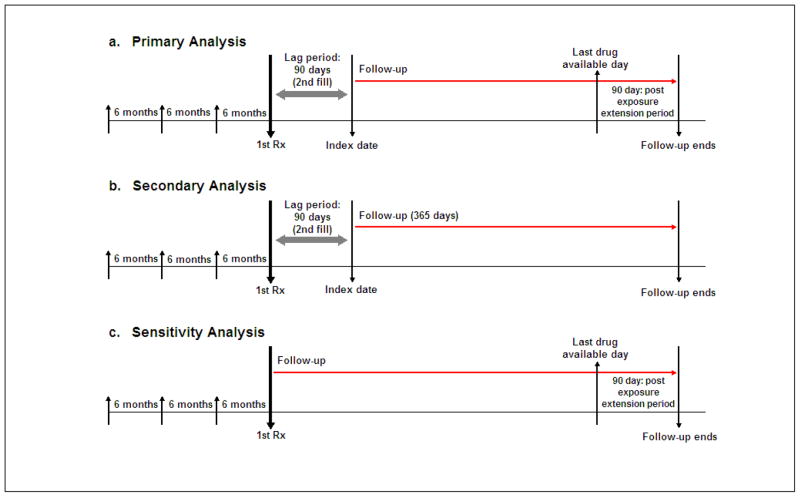
Figure 1. Study Design.
Subjects were required to have at least one claim each during the prior three 6-month intervals. For both the primary (“as treated”) and secondary (“first exposure carried forward”) analyses, follow-up began at the 91st day after filling the first prescription of either exposures of interest. The second prescription fill for the same exposure drug group was required during the 90-day lag period. For the primary analysis (a), we continued the follow-up until the 90 days after the last drug available date. Last drug available date was calculated with a number of days of supply after the last prescription fill date. For the secondary analysis (b), the follow-up continued until 365 days after the index date. Patients were considered “always exposed” for the first exposure drug group during the follow-up period. In a sensitivity analysis (c), follow-up began at the first prescription fill and ended 90 days after the last drug available date.