Summary
Cytokine storm during viral infection is a prospective predictor of morbidity and mortality, yet the cellular sources remain undefined. Here, using genetic and chemical tools to probe functions of the S1P1 receptor, we elucidate cellular and signaling mechanisms important in initiating cytokine storm. While S1P1 receptor is expressed on endothelial cells and lymphocytes within lung tissue, S1P1 agonism suppresses cytokines and innate immune cell recruitment in wild-type and lymphocyte deficient mice, identifying endothelial cells as central regulators of cytokine storm. Furthermore, our data reveal immune cell infiltration and cytokine production as distinct events both orchestrated by endothelial cells. Moreover, we demonstrate that suppression of early innate immune responses through S1P1 signaling results in reduced mortality during infection with a human pathogenic strain of influenza virus. Modulation of endothelium with a specific agonist suggests that diseases where amplification of cytokine storm is a significant pathological component could be chemically tractable.
Introduction
Morbidity and mortality caused by severe influenza infections reflects properties intrinsic to the virus strain, including replication potential, receptor usage and cytopathic effects on pulmonary epithelial cells (Garcia-Sastre, 2010; Tscherne and Garcia-Sastre, 2011). In addition to viral intrinsic factors, host specific traits such as divergent susceptibilities to infection as well as differences in host immune responses may ameliorate or exacerbate both infection and clinical outcome. An overly aggressive innate response, with early recruitment of inflammatory leukocytes to the lung, was a key contributor to the morbidity of the 1918 influenza infection (Kobasa et al., 2007). More recent clinical literature on avian H5N1 infection documented a significant association between excessive early cytokine responses, immune cell recruitment and poor outcome (de Jong et al., 2006). Public health approaches to influenza pandemics have relied primarily on preventative vaccine strategies and supportive measures including the use of antiviral therapies. Nevertheless, the speed at which the 2009 H1N1 influenza virus swine pandemic spread during the lag of vaccine availability highlighted the need to identify additional mechanism for amelioration of influenza virus infection (Openshaw and Dunning, 2010). Anti-viral drugs inhibiting virus replication may select for mutational escape rendering the therapy ineffective. Modulation of the host immune response has the potential advantage of exerting less selective pressure on viral populations. While the prospect of blunting cytokine storm is enticing, a major limitation to treating diseases in which cytokine storm contributes to pathogenesis is the limited understanding of the cellular triggers of this process. Thus we sought to define cellular signaling pathways that are chemically tractable to test the hypothesis that modulating cytokine storm would provide novel insight into influenza pathogenesis with potential therapeutic implications.
Chemical modulators of the sphingosine-1-phosphate (S1P) signaling system have provided insight into immune cell trafficking and immune responses (Rivera et al., 2008; Rosen et al., 2009). Due to the immunomodulatory properties of S1P receptor signaling, chemical agonists have been used successfully for the treatment of relapsing and remitting multiple sclerosis (Brinkmann et al., 2010). While the systems biology of the S1P receptors is complex, with five receptor subtypes, differential expression, coupling, attenuation and catabolism (Rosen and Liao, 2003), the availability of selective chemical probes together with mouse genetic models allow detailed insights into immunopathogenesis. We previously showed that a non-selective S1P receptor agonist inhibited cytokine storm, dendritic cell migration and subsequent antigen-specific T cell proliferation, protecting the lung tissue from host-mediated injury. Alleviation of immunopathology by the non-selective S1P receptor agonist did not affect virus clearance nor the production of neutralizing antibodies, demonstrating that although cytokine storm was diminished, retention of a sufficient cellular and humoral immune response as well as long-term protective immunity still occur (Marsolais et al., 2008; Marsolais et al., 2009).
Here, through the use of an S1P1 receptor subtype selective agonist as well as genetic and biochemical tools, we define a crucial endothelial signaling loop important for the initiation of cytokine storm. We reveal that cytokine secretion and immune cell infiltration are separable events both regulated by the pulmonary endothelium. Further, we demonstrate that suppression of early innate immune responses through S1P1 signaling results in reduced mortality during human pathogenic influenza virus challenge. Thus, S1P1 receptor signaling in endothelium provides a mechanism for attenuation of influenza virus induced morbidity and reveals an unexpected role for endothelial cells as regulators of cytokine storm.
Results
Administration of an S1P1 agonist blunts cytokine storm
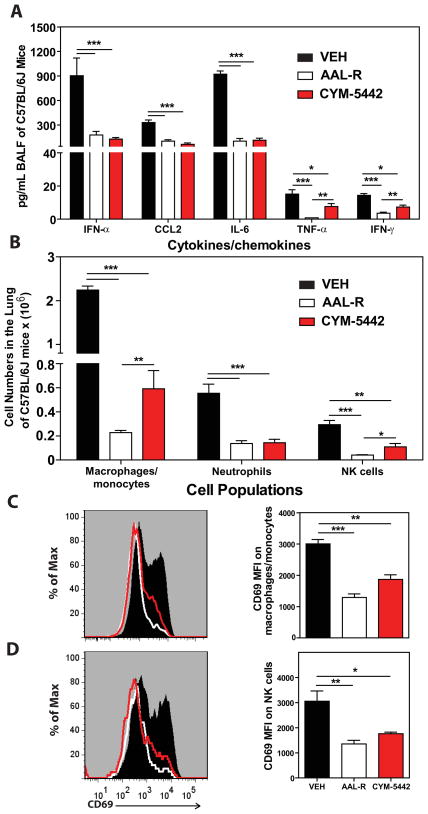
Previously we reported that treatment of influenza virus-infected mice with AAL-R, a promiscuous S1P receptor agonist for S1P1 and S1P3–5 receptors, inhibits early pro-inflammatory cytokine expression and innate immune cell accumulation within the lung (Marsolais et al., 2008; Marsolais et al., 2009). To assess the contribution of S1P1 receptor signaling in inhibition of influenza virus-induced early inflammation, infected mice were treated with the S1P1-receptor specific agonist, CYM-5442 (Gonzalez-Cabrera et al., 2008). Treatment with 2mg/kg of CYM-5442 twice daily significantly inhibited secretion of cytokines and chemokines associated with influenza virus induced pathology including IFN-α, CCL2, IL-6, TNF-α and IFN-γ (Fig. 1A) in addition to CCL3, CCL5, CXCL2 and IL-1α (Fig. S1A) compared to vehicle treated mice 48-hours post-infection. CYM-5442 reduction of IFN-α, CCL2, CCL3, CCL5, IL-1 α, and IL-6 expression was as complete as treatment with the promiscuous agonist, however it was not as effective as AAL-R in suppressing CXCL2, TNF- α and IFN- γ (Fig. 1A and Fig. S1A), suggesting a role for other S1P receptors in modulating those cytokines. In addition to inhibiting cytokine/chemokine production, AAL-R and CYM-5442 administration to influenza-virus-infected mice blunted the accumulation of innate inflammatory infiltrate characterized as macrophages/monocytes (CD11b+, F480+, Ly6G−), neutrophils (CD11b+, LyG6+, F480−), and NK cells (NK1.1+, CD3−), although AAL-R was more effective at inhibiting macrophage/monocyte and NK cell accumulation in the lung (Fig. 1B). We also observed significantly reduced CD69 expression on macrophage/monocytes and NK cells following treatment with either CYM-5442 or AAL-R 48-hours post-infection, demonstrating diminished cell activation (Fig. 1C and D). Despite significant blunting of innate immune cell recruitment and cytokine/chemokine responses, we observed no differences in viral titers following AAL-R or CYM-5442 treatment compared to vehicle treated mice (Fig. S2), demonstrating that S1P1 agonism neither inhibits nor enhances viral replication. Collectively, these results reveal that activation of a single sphingosine-1-phosphate receptor, S1P1, is sufficient to blunt global innate inflammation following mouse adapted influenza virus infection in mice.
Figure 1.
S1P1 receptor agonism suppresses early pro-inflammatory cytokine/chemokine production and innate immune cell recruitment during influenza virus infection. Mice were infected with 1 × 104 PFU WSN influenza virus and either Vehicle (water), AAL-R (0.2mg/kg) (1 hour post-infection) or CYM5442 (2mg/kg) (1,13, 25 and 37 hours-post-infection) were administered i.t. to mice. (A) Pro-inflammatory cytokines and chemokines were measured 48 hours post-infection in BALF by ELISA. (B) Total numbers of innate immune cells were quantified from collagenase digested lungs by flow cytometry at 48 hours post-influenza virus infection. (C) Histograms (left) and mean fluorescent intensity (MFI) (right) of CD69 expression on macrophages was quantified on vehicle, AAL-R or CYM-5442 treated mice 48 hours post-influenza virus infection by flow cytometry staining. (D) Histograms (left) and MFI (right) of NK cell CD69 expression quantified as done in C. Data represent average ± SEM from 4–5 mice/group. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Results are representative of greater than 6 independent experiments.
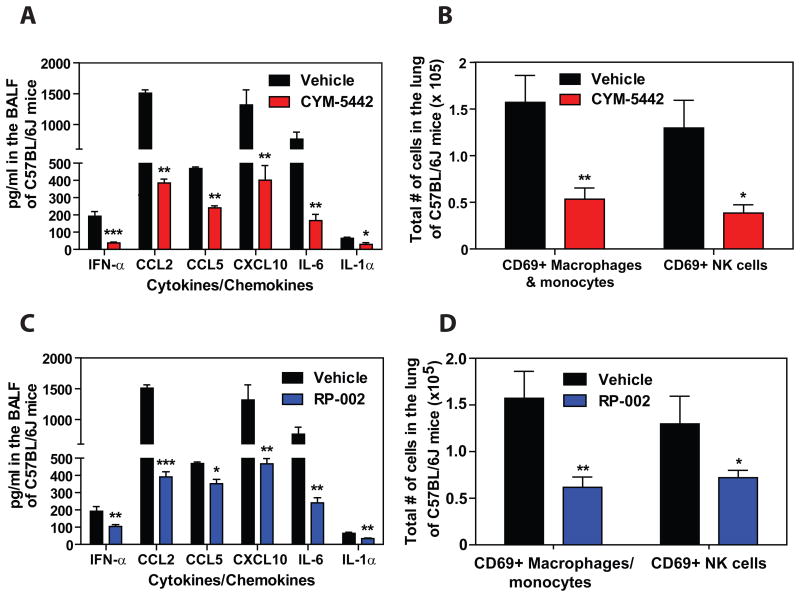
We next asked whether S1P1 receptor agonism could suppress early innate cytokine and chemokine responses following infection with a human pathogenic isolate of influenza virus. To test this we infected mice with a virulent isolate of the 2009 H1N1 pandemic influenza virus that was isolated from a hospitalized patient and has never been passaged through mice (A/Wisconsin/WSLH34939/09) (Itoh et al., 2009). Infection of C57BL/6J mice with this strain causes severe disease resulting in rapid mortality beginning between days 4 & 6 post-infection (data not shown). Similar to infection with mouse adapted WSN influenza virus, treatment with CYM-5442 following infection with H1N1:2009 significantly suppressed cytokine and chemokine responses and the accumulation of activated innate immune cells 48 hours post-infection (Fig. 2A & B). We also tested an additional S1P1-receptor selective agonist (RP-002) to provide support for S1P1 receptor specificity and to show directly that S1P1-selective agonists share this modulation of innate immunopathology. We infected mice with H1N1:2009 pandemic influenza virus as above and treated mice with RP-002 at 1 and 25 hours post-infection. Similar to what we observed with CYM-5442, RP-002 treatment significantly inhibited the production of multiple pro-inflammatory cytokine and chemokines (Fig. 2C) and suppressed the accumulation of activated (CD69+) macrophages/monocytes and NK cells in the infected lung 48 hours post-infection (Fig. 2D). Moreover, RP-002-mediated suppression of innate immune cell recruitment and cytokine/chemokine production occurred without altering lung viral titers (Fig. S3) demonstrating that S1P1 agonist mediated suppression of cytokines and chemokines is not due to direct effects on influenza virus replication.
Figure 2.
S1P1 receptor agonism suppresses early pro-inflammatory cytokine/chemokine production and recruitment of activated innate immune cells during human pathogenic H1N1:2009 swine influenza virus infection. Mice were infected with 1 × 105 PFU A/Wisconsin/WSLH34939/09 influenza virus and either Vehicle (water), CYM5442 (2mg/kg) (1, 13, 25 and 37 hours-post-infection) or RP-002 (2mg/kg on 1 and 25 hours post-infection) were administered i.t. to mice. Pro-inflammatory cytokines and chemokines were measured 48 hours post-infection in BALF by ELISA in either CYM-5442 (A) or RP-002 treated mice (C). Total numbers of innate immune cells were quantified from collagenase digested lungs by flow cytometry at 48 hours post-influenza virus infection in mice treated with either CYM-5442 (B) or RP-002 (D). Data represent average ± SEM from 4–5 mice/group. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Results are representative of 2 independent experiments.
S1P1 agonist mediated suppression of early innate immune responses results in protection to human pathogenic influenza virus challenge in mice
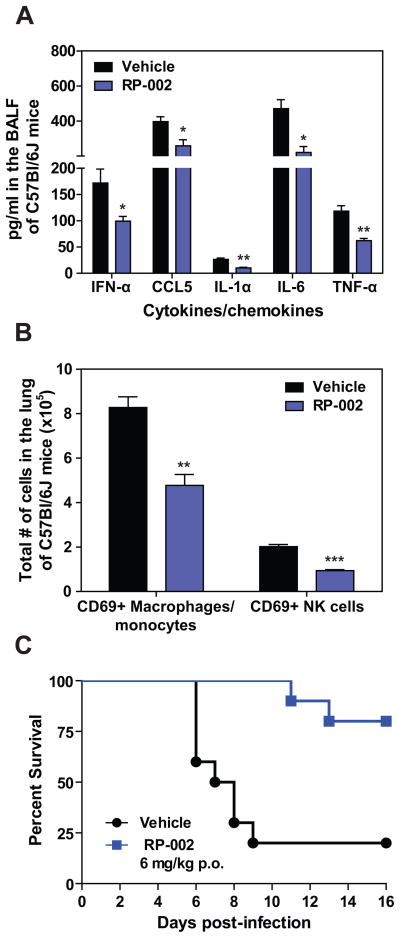
Early dysregulated innate immune responses in the lung have been associated with morbidity and mortality during infection with highly pathogenic strains of influenza virus (Cilloniz et al., 2009; Kobasa et al., 2007). Therefore we asked whether blunting early innate cytokine/chemokine responses using an S1P1 agonist could protect mice from lethal infection with a virulent human isolate of pandemic 2009 H1N1 that had not been passaged in mice (A/Wisconsin/WSLH/34939/09). In order not to impose additional stress on the infected lung and because oral delivery is a popular route for drug administration, we orally administered the S1P1 agonist, RP-002, at 6mg/kg by gavage to C57BL/6J mice infected with 1 × 105 PFU of A/Wisconsin/WSLH/34939/09 at 1 and 25 hours post-infection. RP-002 significantly suppressed cytokine amplification (Fig. 3A) as well as recruitment of myeloid cells to the infected lung, as measured by flow cytometry (Fig. 3B).
Figure 3.
Oral administration of an S1P1 agonist suppresses early innate cytokine and chemokine production and significantly improves survival to lethal infection with H1N1 2009 swine influenza virus. Mice were infected with 2 × 105 PFU A/Wisconsin/WSLH34939/09 and treated by gavage with either Vehicle (water), or RP-002 (6mg/kg on 1 and 25 hours post-infection, for cytokine and cell recruitment assays at 48 hours, while a third dose was administered at 49 hours for the 16 day survival experiment). (A) Levels of pro-inflammatory cytokines and chemokines were analyzed in the BALF 48 h post-infection by ELISA. (B) Total numbers of activated (CD69+) macrophages/monocytes and NK cells were detected in collagenase digested lungs 48 h post-infection by flow cytometry. (C) Mice were monitored daily for survival for 16 days post-infection. For (A) and (B) data represent the average ± SEM of 4–5 mice/group, while (C) had 10 mice per group. *, p < 0.05; **, p< 0.005; ***, p < 0.0005. Data is representative of three independent experiments.
Early administration of RP-002 resulted in enhanced survival time after lethal challenge with A/Wisconsin/WSLH/34939/09, with death initiating in vehicle treated mice on day 6 post-infection compared to day 11 post-infection for RP-002 treated mice (Fig. 3C, p< 0.005). Moreover, RP-002 treatment resulted in significant improvement in overall survival compared to vehicle treated mice (20% mortality in RP-002 vs. 80% mortality for vehicle, p < 0.005) (Fig. 3C). These findings demonstrate a significant biological phenotype resulting from early S1P1 receptor agonist treatment following pathogenic influenza virus infection. Further, the fact that suppression of early innate immune responses results in improved survival to infection with human pathogenic strains of influenza virus expands previous observations of an association between humans and animal models with dysregulation of innate immune responses contributing to morbidity and mortality by now showing directly for the first time that aborting an early step in cytokine amplification significantly protects against an ordinarily lethal human pathogenic H1N1:2009 infection. Importantly, treatment with RP-002 ameliorated morbidity and mortality associated with human pathogenic H1N1:2009 severe influenza infection. Effects upon survival are in keeping with our previously published data (Marsolais et al, 2009) that this mechanism does not alter virus-specific neutralizing antibody response, affinity maturation and class switching. This documents that the cytokine storm plays a direct and cardinal role in influenza-mediated lung disease. We then sought to delineate the mechanism of S1P1 suppression of cytokine amplification.
S1P1 is expressed on pulmonary endothelium and lymphocytes
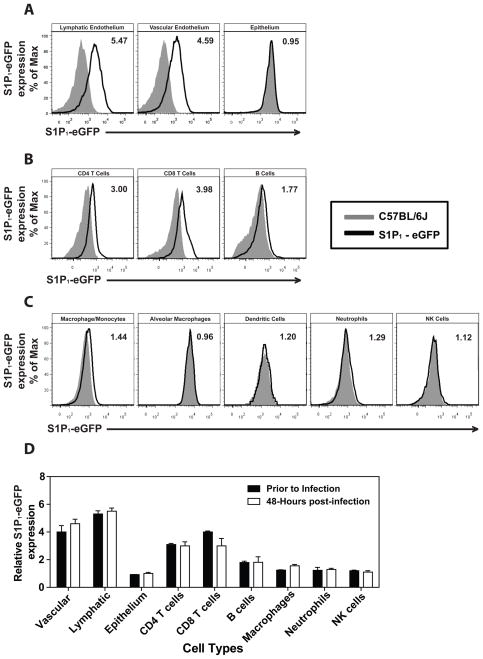
To identify cells in the lung that express the S1P1 receptor, we utilized an eGFP-S1P1 receptor knock-in mouse where the native S1P1 receptor was homologously replaced with a functional enhanced green fluorescent protein (eGFP) tagged S1P1 receptor (S1P1-eGFP) (Cahalan et al., 2011). This mouse model allowed direct detection of eGFP-S1P1 receptor protein expression by flow cytometry with additional biochemical confirmation using highly specific antibodies for GFP followed by Western blotting that distinguishes the fused S1P1-eGFP receptor by specific molecular weight. High levels of S1P1-eGFP were detected on lung lymphatic (CD45−, CD31+, gp38+) and vascular (CD45−, CD31+, gp38−) endothelium while pulmonary epithelium (CD45−, CD31−, EpCAM+) was negative for S1P1-eGFP (Fig. 4A). As reported previously, CD4+ T cells (CD4+, CD3+), CD8+ T cells (CD4+, CD3+), and B cells (B220+, CD19+) expressed S1P1-eGFP (Fig. 4B), while leukocytes including macrophages/monocytes, a subset of alveolar macrophages (F480+, CD11c+, CD11b−), dendritic cells (CD11c+, I/A-I/E+, CD205+, F480−), neutrophils, NK cells (NK1.1+, CD3−) and innate lymphoid cells (Lin−Sca1+) expressed negligible levels of S1P1-eGFP (Fig. 4C and data not shown). Cells from uninfected mice expressed similar levels of S1P1-eGFP as infected mice demonstrating that S1P1 receptor expression is not altered following influenza virus infection (Fig. 4D). To confirm the S1P1-eGFP expression pattern, a Western blot for S1P1-eGFP was performed on cells FACS sorted from the lungs of infected and uninfected mice. Endothelial cells, T cells and B cells were positive for S1P1-eGFP in uninfected mice, confirming our flow cytometry results (Fig. S4A). However, while S1P1-eGFP was detected by Western blot in B cells from uninfected mice, we did not detect S1P1-eGFP expression by Western Blot in influenza virus infected lung-resident B cells (Fig. S4A). We did not detect S1P1-eGFP expression by Western blot in epithelial cells, macrophages/monocytes, and alveolar macrophages either before or after influenza virus infection (Fig. S4A). Moreover, CYM-5442 treatment of infected S1P1-eGFP knock in mice did not diminish expression of S1P1-eGFP on endothelial cells, T cells or B cells, demonstrating that administration of this S1P1-specific agonist does not induce degradation of the S1P1 receptor (Fig. S4B). Therefore, CYM-5442 does not exert an antagonistic effect due to receptor degradation, demonstrating functional agonism of S1P1 as the mechanism by which CYM-5442 suppresses cytokine storm.
Figure 4.
S1P1 receptor is expressed on pulmonary endothelium and lymphocytes but not on pulmonary epithelial cells. Flow cytometry histograms showing eGFP fluorescence from heterozygous S1P1-eGFP on lung endothelial and epithelial cells mice, (A) CD4, CD8 T cells and B cells (B) and macrophages, neutrophils, dendritic cells and NK cells (C), prior to influenza virus infection. (D) Relative expression of S1P1-eGFP on lung cell populations before or 48-hours after influenza virus infection. The numbers in the upper right corner of each plot represents the relative eGFP expression as calculated by dividing the MFI of eGFP on S1P1-eGFP transgenic mice over eGFP MFI of C57BL/6J littermate controls. Data represent average SD from 2–5 mice/group. Results are representative of 3 independent experiments.
Inhibition of cytokine storm is not due to lymphocyte S1P1 receptor activation
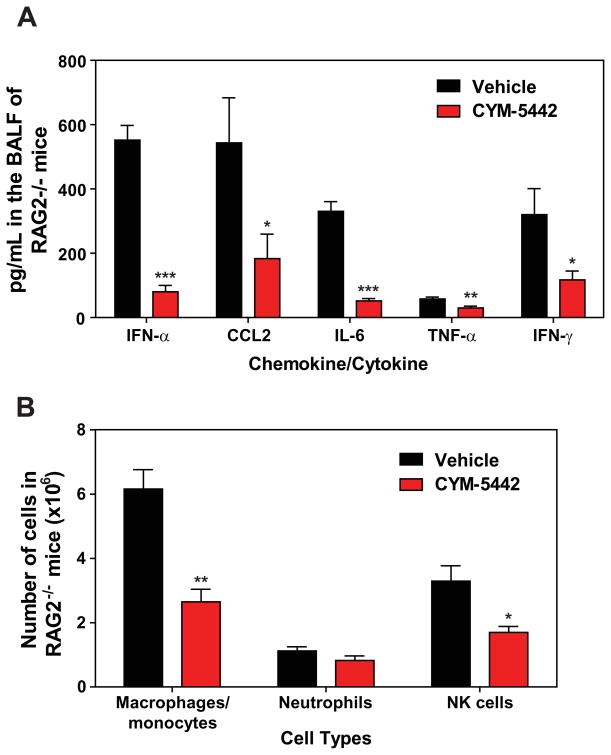
Lymphocytes and pulmonary endothelium are the only cells within the lung that express measurable amounts of S1P1-eGFP as detected by flow cytometry and western blot. It is plausible that lymphocytes within the lung provide a bystander effect during the innate immune response to influenza virus infection. To rule out a role for lymphocytes during S1P1 receptor-mediated inhibition of inflammation following influenza virus infection, lymphocyte deficient Rag2−/− mice were infected and treated with vehicle or CYM-5442. Administration of CYM-5442 to influenza virus-infected Rag2−/− mice resulted in significant inhibition of expression of IFN-α, CCL2, IL-6, TNF-α and IFN-γ (Fig. 5A) in addition to CCL5, CXCL2 and CXCL10 (Fig. S1B). Atypical lymphocyte populations that could in principal contribute to suppression of cytokine production did not express S1P1-eGFP and thus also do not contribute to this mechanism. Inflammatory cell infiltrates, including macrophages/monocytes and NK cells were significantly reduced in CYM-5442 treated Rag2−/− mice (Fig. 5B). Reduced numbers of neutrophils were also observed within the infected lung, but the difference was not statistically significant (Fig. 5B). Moreover, lung infiltrating macrophages and NK cells in Rag2−/− mice were less activated, as measured by CD69 surface expression (Fig. S5). CYM-5442 treatment of influenza virus-infected lymphocyte-deficient mice inhibits the innate inflammatory response, excluding a role for lymphocytes as key regulators of influenza virus-induced cytokine storm.
Figure 5.
S1P1 receptor agonism suppresses early pro-inflammatory immune responses independently of lymphocytes. Rag2−/− mice were infected and treated with either vehicle or CYM-5442 as done in Figure 1. (A) Pro-inflammatory cytokines and chemokines were measured in BALF by ELISA 48-hours post-influenza infection. (B) Total numbers of innate immune cell recruitment were quantified from collagenase digested lungs by flow cytometry at 48 hours post-influenza virus infection. Data represent average ± SEM from 5 mice/group. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Results are representative of two independent experiments.
S1P1 receptor agonism suppresses immune cell recruitment through down-regulation of chemokine production by lung endothelial cells
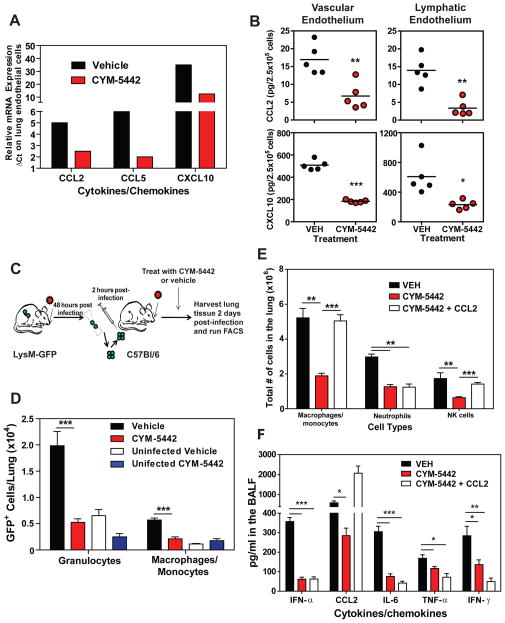
The strong expression of S1P1-eGFP receptor on pulmonary endothelium, coupled with the inhibition of cytokine storm in RAG2−/− mice, suggests that S1P1 receptor signaling in lung endothelial cells suppresses cytokine storm. Endothelial cells have been shown to produce various cytokines and chemokines during inflammatory processes and may be a source of cytokines and chemokines in the lung during influenza virus infection. To evaluate the effects of CYM-5442 treatment on pulmonary endothelial cell cytokine and chemokine production we purified lung endothelial cell populations and performed mRNA and protein analyses. Endothelial cells expressed elevated levels of the chemokines CCL2, CCL5 and CXCL10 at the mRNA level (Figs. 6A). Importantly, treatment with CYM-5442 significantly reduced mRNA expression of chemokines in lung endothelial cells compared to vehicle treated mice early after influenza virus infection (Fig. 6A). Examination of chemokine production in purified lung vascular and lymphatic endothelial cells revealed a significant reduction in chemokine production at the protein level after CYM-5442 treatment (Fig. 6B). Analysis of pulmonary endothelial cell integrin expression did not show appreciable differences in mice infected with influenza virus treated with vehicle compared to CYM-5442 (Fig. S6).
Figure 6.
S1P1 receptor agonism actively suppresses recruitment of innate immune cells through down-regulation of chemokine expression on lung endothelial cells. (A) Relative mRNA expression of chemokines from purified lung endothelial cell populations at 36-hours post influenza virus infection. (B) Protein expression of CCL2 and CXCL10 measured by ELISA on FACS purified vascular and lymphatic lung endothelial cells 48 hours post influenza virus infection. (C) LysM-GFP mice were infected with 1 × 104 PFU of influenza virus. Two days later bone marrow cells were harvested from infected LysM-GFP mice and transferred into C57BL/6J mice that were either left uninfected or infected with 1 × 104 PFU of influenza virus 2-hours prior. Mice receiving bone marrow cells from LysM-GFP positive mice were treated with either vehicle or CYM-5442 (2mg/kg 1, 13, 25, and 37 hours post-infection) and 48-hours post-infection total lung cells were harvested from lung homogenates and the total numbers of GFP expressing granulocytes (CD11b+, Ly6G+) and macrophages/monocytes (CD11b+F480+) were quantified. (D) Shows the total number of LysM-GFP positive granulocytes and macrophages/monocytes per lung of uninfected or infected mice treated with either vehicle or CYM-5442. Exogenous intratracheal administration of chemokine restores recruitment of innate immune cells however, does not resurrect cytokine/chemokine production after CYM-5442 treatment. (E) Mice were infected with 1 × 104 PFU WSN influenza virus and either Vehicle (water), or CYM5442 (2mg/kg 1,13, 25 and 37 hours-post-infection) were administered to mice i.t.. Following administration of CYM-5442, recombinant mouse CCL2 was administered (50μg) i.t. directly into the lungs. The bar graph shows the total numbers of macrophages/monocytes, neutrophils and NK cells in the lung 48 hours post-influenza virus infection. (F) Pro-inflammatory cytokines and chemokines were measured in BALF 48 hours post-infection by ELISA in mice treated with vehicle, CYM-5442 or CYM-5442 + rmCCL2 as in E. Data represent average SEM from 5 mice/group. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Results are representative of two independent experiments.
Because chemokine presentation on endothelial cells is crucial for leukocyte activation and extravasation into infected tissue, we asked whether reduction of chemokine expression following CYM-5442 treatment resulted in reduced entry of inflammatory cells into the infected lung. For these experiments we used LysM-GFP mice in which macrophages, monocytes and neutrophils express green fluorescent protein, allowing us to track these cell populations after adoptive transfer into congenic C57BL/6J mice. We infected LysM-GFP mice with influenza virus and 48 hours later purified bone marrow cells for adoptive transfer. 5 × 106 LysM-GFP bone marrow cells were transferred into influenza virus infected C57BL/6J mice treated with either vehicle or CYM-5442 (Fig. 6C). The lungs of infected or uninfected C57BL/6J mice receiving LysM-GFP cells were then harvested 48 hours post-infection and the numbers of GFP positive cells in the lung were quantified. Treatment with CYM-5442 resulted in significantly reduced recruitment of both GFP positive neutrophils (CD11b+ F480− Ly6G+) and macrophages/monocytes (CD11b+ Ly6G− F480+) into the lung (Fig. 6D). Therefore, CYM-5442 treatment inhibits the infiltration of circulating inflammatory cells into the lung.
S1P1 receptor signaling is associated with enhanced capillary integrity (Rosen et al., 2007), where S1P1 receptor antagonists induce vascular leakage (Rosen et al., 2008; Sanna et al., 2006), and S1PR-selective agonists protect from vascular leakage induced by exogenous administration of VEGF (Sanchez et al., 2003). While myelomonocytic recruitment is not modulated by endothelial permeability changes, we formally assessed whether suppression of inflammatory cell recruitment to the lung reflected a passive inhibition of leukocyte recruitment or whether suppression of chemokines on lung endothelial cells limits innate immune cell recruitment. Rescue experiments were performed where mice were infected with influenza in the presence or absence of intratracheal administration of recombinant murine CCL2 (rmCCL2), with or without CYM-5442 treatment. Administration of rmCCL2 to infected mice treated with CYM-5442 restored macrophage/monocyte and NK cell numbers in the lung to levels equivalent to infected mice treated with vehicle (Fig. 6E) 48 hours post-infection. These data demonstrate that CYM-5442 inhibition of cellular infiltration into the lung is not due to the enhancement of endothelial cell barrier function but due to suppression of chemokine production. Therefore, S1P1 receptor agonism suppresses endothelial cell chemokine expression, resulting in diminished cell infiltration. Despite the rescue of CCL2 levels in the BALF and the restoration of macrophage/monocyte recruitment into the lung of CYM-5442-treated mice, global cytokine and chemokine production was not restored (Fig. 6F and Fig. S1C). Influenza virus infection of CCR2 deficient mice results in substantial reductions of infiltrating macrophages/monocytes (Dawson et al., 2000). To rule out infiltrating macrophages we infected CCR2 deficient mice with WSN influenza virus as done above and treated with either vehicle or CYM-5442. Infection of CCR2 deficient mice resulted in significantly reduced numbers of macrophages/monocytes without altering neutrophil or NK cell numbers in the lung 48 hours post-infection (Fig. S7A). Despite reduced levels of macrophages/monocytes in the lung we still detected significant levels of IFN-α, CCL2, CCL3, CCL5, CXCL2, CXCL10, IL-1α, IL-6 and IFN-γ (Fig. S7B). More importantly, treatment of CCR2 deficient mice with CYM-5442 still significantly reduced all cytokines and chemokines tested at 48 hours post-infection except for CCL5 (Fig. S7B). Failure to inhibit cytokine and chemokine production in CCR2 deficient mice or to re-establish cytokine and chemokine production by the induction of macrophage/monocyte cell infiltration through rmCCL2 administration following CYM-5442 treatment, suggests that monocytes and macrophages are not major sources of early cytokines and chemokine production. Furthermore, these data indicate that cell recruitment and cytokine responses may be uncoupled events.
Pro-inflammatory cytokine responses are independent of innate immune cell recruitment and dependent on type I Interferon signaling
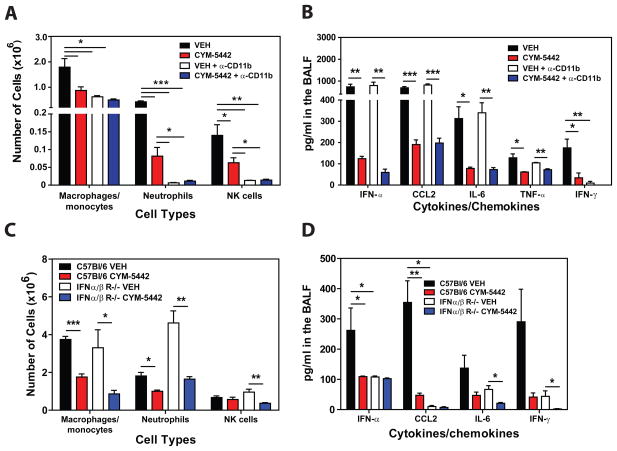
Infiltration of macrophages and NK cells alone does not appear to be associated with cytokine storm. Therefore we evaluated the contribution of total inflammatory cell infiltration to cytokine production during influenza virus infection. Mice were treated with an anti-CD11b antibody (M7/80) which has been shown to inhibit the recruitment of CD11b expressing cells into inflamed tissues (Rosen and Gordon, 1987). While treatment with anti-CD11b significantly inhibited the recruitment of macrophages/monocytes, neutrophils and NK cells into the lung (Fig. 7A), we observed no reduction in levels of pro-inflammatory cytokines/chemokines, other than IFN-γ (Fig. 7B), which is probably a direct result of diminished infiltration of NK cells, the primary source of IFN-α at this time point. Moreover, treatment with CYM-5442 significantly inhibited the production of IFN-α, CCL2, IL-6, TNF-α and IFN-γ (Fig. 7B) in addition to CCL3, CCL5, CXCL2, CXCL10 and IL-1α (Fig. S1D) in anti-CD11b treated mice. These results further demonstrate that innate cell recruitment and cytokine production are independent events early after influenza virus infection both which are inhibited by S1P1 receptor agonism of pulmonary endothelial cells. Importantly, our data demonstrates that inflammatory cell infiltration into the lung is not required for cytokine and chemokine production. An additional line of evidence excluding hematopoietic cells from S1P1 agonist-mediated reduction of early innate cytokine and chemokine responses following influenza virus infection was provided by irradiation-resistance of S1P1 agonist suppression. C57BL/6J mice were irradiated with 500 rads and 24 hours later infected with WSN influenza virus and treated with either vehicle or CYM-5442. Irradiation reduced CD45+ hematopoietic cells in lung by 90% (Vehicle: 2 × 107 cell/lung vs. 500 rads: 2 × 106 cells/lung) and importantly, treatment of irradiated mice with CYM-5542 significantly reduced the levels of IFN-α (irradiated vehicle: 86 pg/mL vs. irradiated CYM-5442: 17.9pg/mL, p = 0.0006), CCL2 (irradiated vehicle: 944 pg/mL vs. irradiated CYM-5442:452 pg/mL, p = 0.00002), CXCL10 (irradiated vehicle: 204 pg/mL vs. irradiated CYM-5442: 83 pg/mL, p = 0.00003) and IL-6 (irradiated vehicle: 170 pg/mL vs. irradiated CYM-5442: 70 pg/mL, p = 0.0001), demonstrating that a radiation resistant cell population mediated S1P1 receptor mediated suppression of the inflammatory response. These results, coupled with the restricted S1P1 receptor expression pattern, support an important role for endothelial cells in regulating pulmonary innate immune responses to influenza virus.
Figure 7.
Pro-inflammatory cytokine responses are independent of innate immune cell recruitment and dependent on Type I Interferon signaling. (A) Total numbers of innate immune cells were quantified from lung digests by flow cytometry at 48 hours post-influenza virus infection in mice treated 1 hour post-infection with vehicle or CYM-5442 in the presence of either anti-CD11b or isotype control antibody (M7/80) (0.5mg/mouse) 0 and 24 hours post-infection. (B) Pro-inflammatory cytokines and chemokines were measured 48 hours post-infection by ELISA in BALF in mice treated as in A. C57BL/6J or IFN- αβ receptor deficient mice were infected with 1 × 104 PFU of influenza virus and treated with either vehicle or CYM-5442 (2mg/kg 1, 13, 25, and 36 hours post infection. (C) Total numbers of innate immune cells were quantified from lung digests by flow cytometry at 48 hours post-influenza virus infection and (D) Pro-inflammatory cytokines and chemokines were measured 48 hours post-infection by ELISA in BALF fluid. Data represent average ± SEM from 5 mice/group. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Results are representative of two independent experiments.)
Type I interferons, predominantly IFN-α species, are elevated early after respiratory virus infection and are thought to be crucial for the production of pro-inflammatory cytokines and chemokines. Our results thus far have demonstrated that CYM-5442 treatment consistently inhibits the production of IFN-α in the lung early after influenza virus infection (Figs. 1, 2, 5 and 6). Therefore we postulated that blunting IFN-α production may be a mechanism by which CYM-5442 inhibits cytokine storm early after influenza virus infection. To address this we infected IFNα/β receptor knockout mice with influenza virus, treated these mice with either vehicle or CYM-5442 and measured innate cell recruitment and cytokine/chemokine production 48 hours post-infection. Significant differences were not observed in inflammatory cell recruitment of macrophages/monocytes, neutrophils and NK cells into the lung of IFNα/β receptor deficient mice when compared to C57Bl/6J mice (Fig. 7C). As seen with infected C57BL/6J mice, CYM-5442 treatment inhibited innate immune cell recruitment in IFNα/β receptor deficient mice 48-hours post-infection (Fig. 7C). Despite recruitment of innate immune cells into the lung of IFNα/β receptor deficient mice 48 hours post-infection, these mice exhibited significantly reduced levels of IFN-α, CCL2, IL-6 and IFN-γ (Fig. 7D) in addition to CCL5 and CXCL10 in the BALF fluid (Fig. S1E). Taken together, our data demonstrate that regulation of cellular recruitment into the pulmonary tissue is mediated by endothelial cells and is independent of Type I Interferon signaling. Moreover, S1P1 receptor agonism of endothelial cells inhibits IFN-α production and results in the dampening of global pro-inflammatory cytokine responses.
Discussion
The recruitment of innate immune cells into the lungs combined with excessive pro-inflammatory cytokine and chemokine production are hallmarks of influenza virus infection (La Gruta et al., 2007). Contrary to current dogma, which places lung epithelium and inflammatory infiltrate at the center of influenza virus induced cytokine storm (La Gruta et al., 2007), we demonstrate here for the first time a central role for pulmonary endothelium in regulating this process. Furthermore, we determined that both innate immune cell recruitment and early innate cytokine and chemokine production are uncoupled events, with endothelial cells at the center of both processes. The chemical and genetic approaches shown here have a potentially broad impact on understanding disease pathogenesis. Early dysregulation of innate cellular and cytokine responses predict disease severity and death during highly pathogenic influenza virus infection (Bermejo-Martin et al., 2009; Cilloniz et al., 2009; de Jong et al., 2006; Kobasa et al., 2007; Kobasa et al., 2004). The early induction of the cytokines; IFN-α, TNF-α, IL-1α, and IL-6 and chemokines; CCL2, CCL3, CXCL2 (IL-8) and CXCL10 are associated with symptom formation in humans (Hayden et al., 1998; Kaiser et al., 2001). TNF-α, IL-1 and IL-6 possess multifunctional activities and are associated with morbidity during influenza virus infection. Chemokines such as CCL2, CCL3, CXCL2 and CXCL10 induce the recruitment of innate immune cells into the lung which can release more cytokines exacerbating cytokine storm and further damage the lung. We demonstrate that suppression of early innate immune responses through S1P1 signaling on endothelial cells results in significantly reduced mortality during infection of mice with a human pathogenic strain of influenza virus (Fig. 3). Importantly, our results identify a novel pulmonary cell type, endothelial cells, as potential targets for suppressing excessive innate inflammatory responses.
The ability of CYM-5442 to diminish the production of IFN-α early following influenza virus infection is broadly relevant. The requirement of type I interferon signaling for the early production of multiple cytokines and chemokines after influenza virus infection is striking. Moreover, production of pro-inflammatory cytokines requires type I interferon signaling, but the type I Interferon pathway is dispensable for cell recruitment. While type I interferon signaling is well known to inhibit viral replication (Garcia-Sastre and Biron, 2006), evidence also points to pathogenic roles for IFN-α during viral infection. Several pro-inflammatory cytokines and chemokines are downstream of type I interferon receptor signaling. Moreover, disease onset correlates directly with local respiratory production of IFN-α in humans (Hayden et al., 1998). Thus type I interferon signaling may play dual roles in viral pathogenesis and viral clearance. S1P1 receptor signaling on pulmonary endothelial cells in vivo blunts but does not abolish IFN-α production and may explain why pathology is lessened without compromising the host’s ability to clear the virus.
The ability of S1P1 receptor activation to suppress cytokine and chemokine production in the lung in irradiated mice, coupled to the pattern of S1P1 receptor expression in the lung, strongly indicates a central endothelial component to early innate immune responses. An interesting question is whether lung lymphatic, vascular or both endothelial cell populations are directly responsible for the observed immune modulation. Moreover, of the large receptor reserve in blood endothelium ensures that S1P1 receptor expression is maintained on both basolateral and luminal surfaces of the targeted endothelial cell populations (Cahalan et al., 2011) and retains a responsiveness to agonist. Additional approaches to separate blood and lymphatic contributions are still needed. While we know that endothelial cells produce chemokines, endothelial-mediated regulation of cytokine production in the lung still needs to be determined. It is possible that endothelial cells may regulate cytokine production in the lung through a complex cross-talk mechanism with lung epithelium or resident hematopoietic cells. Nevertheless, the identification of endothelial cells as central orchestrators of early innate immune mediated inflammation is of fundamental interest and has broad implications for treating multiple diseases. The contribution of aberrant pro-inflammatory cytokine and chemokine production to pathogenesis has been reported for viral and bacterial diseases including; HIV (Stacey et al., 2009), Hantavirus (Borges et al., 2006), SARS (Thiel and Weber, 2008) and pneumococcal bacterial pneumonia (Bergeron et al., 1998). Further, the etiology of several autoimmune conditions has been directly associated with excessive innate immune responses(Kawane et al., 2010; Link, 1998). Thus understanding the cellular pathways that regulate cytokine storm and developing appropriate chemical signaling tools to identify pathophysiological points of control provides not only insight into microbial-host interactions but may ultimately reveal additional approaches to achieve effective immunotherapy in multiple diseases. In addition, our results present a non-lymphopenic mechanism by which sphingosine analog treatment suppress pathogenic immune responses. Lastly, these data suggest that endogenous S1P acting on endothelial S1P1 receptor could be a negative regulator of cytokine amplification and raises the possibility that heterogeneities in S1P metabolism between individuals could contribute to the advantages or disadvantages conferred by genetic individuality to host survival in a number of diseases.
Experimental Procedures
Mice, virus, compounds and reagents
6–8 week old C57Bl/6 male mice and S1P1-eGFP mice described elsewhere (Cahalan et al., 2011) were bred and maintained in a closed breeding facility at The Scripps Research Institute. Influenza A/WSN/33 (WSN; H1N1) and the human H1N1 2009 isolate, A/Wisconsin/WSLH34939/09 (a kind gift from Yoshihiro Kawaoka, University of Madison, Wisconsin) were amplified and plaqued on Madin-Darby Canine Kidney (MDCK) cells. Mice were infected intratracheally (i.t.) with 1×104 PFU of influenza A/WSN/33 virus or intranasally with 1 ×105 PFU of A/Wisconsin/WSLH34939/09 under isoflurane anesthesia. One hour post infection, mice were anesthetized by isoflurane inhalation for i.t. delivery of vehicle (100 ul of water), AAL-R (0.2 mg/kg dissolved in water), CYM-5442 (2 mg/kg dissolved in water) or RP-002 (3mg/kg i.t. or 6mg/kg orally dissolved in water). Multiple doses of compund were administered at the specific times listed in the figurte legends. (AAL-R and CYM-5442 were synthesized according to published methods (Jo et al., 2005). RP-002, (R)-2-(4-(5-(3-cyano-4-isopropoxyphenyl)-l,2,4-oxadiazol-3-yl)-2,3-dihydro-lH-inden-l-ylamino)-N, N-dimethylacetamide hydrochloride, was synthesized according to the published method (Martinborough et al., 2011). The compound had an EC50 for S1P1 of 0.13nM, was >100-fold selective versus S1P5 and 10,000-fold selective versus S1P2, 3 and 4 respectively, when assayed as described (Cahalan et al, 2011). Recombinant murine CCL2 (rmCCL2) was purchased from Shenandoah Biotechnology Inc. (Warwick, PA).
Cytokine and chemokine analysis
The trachea of euthanized mice was exposed, transected, and intubated with a blunt 18 gauge needle. 1 ml of phosphate buffered saline supplemented with Complete Mini, EDTA-free Protease Inhibitor Cocktail (Roche) was infused and recovered 4 times. The recovered bronchoalveolar lavage fluid was spun at 3000 × g for 3 min at 4°C and stored at −80° C until use. Multiplex ELISA was performed on supernatant by Quansys Biosciences (Logan, UT) to detect IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, TNF-α, MIP-1α, MCP-1, GM-CSF, IFN-γ, and RANTES. ELISAs were also performed using CCL2 (MCP-1), CCL5 (RANTES), CXCL10 (IP-10), IL-1α, IL-6, TNF-α and IFN-γ Duoset kits (R & D systems) as well as the VeriKine™ Mouse Interferon-Alpha and Interferon-Beta ELISA Kits (Pestka Biomedical Laboratories, Inc). For the quantification of mRNA chemokine expression, lung endothelial cells were FACS purified to >90–95% purity and mRNA was purified using the RNeasy mini kit from Qiagen. Prior to real time PCR, genomic DNA was digested and cDNA was made using the RT2 First Strand Kit (C-03) according to manufacturer’s instructions (SA Biosciences, Frederick, MD). cDNA (200ng) was added to individual wells and quantified using the RT2 profiler PCR Array according to the manufacturer’s instructions (SA Biosciences, Frederick, MD).
Cellular analysis by flow cytometry
Lungs were harvested from PBS-perfused mice and mechanically diced into small tissue pieces using surgical scissors. Diced lungs were suspended in 4 ml of CDTI buffer [0.5 mg/ml collagenase from Clostridium histolyticum Type IV (Sigma), 0.1 mg/ml Dnase I from bovine pancreas grade II (Roche), 1 mg/ml Trypsin inhibitor Type Ii-s (Sigma) in DMEM] for 1 hr at 37° C. Lung was then disrupted mechanically through a 100 m filter, and red blood cells were lysed using red blood cell lysis buffer (0.02 Tris-HCL and 0.14 NH4Cl). Inflammatory cells were purified by centrifugation in 35% PBS-buffered Percoll™ (GE Healthcare Life Sciences) at 1500 rpm for 15 min. Cell pellets were resuspended in staining buffer and Fc receptors were blocked using 25 μg/ml anti-mouse CD16/32 (BD Biosciences). Cells were stained with the following anti-mouse antibodies: AlexaFluor 488-conjugated gp38 (eBioscience; clone eBio8.1.1), PE-conjugated (BioLegend, Inc.; clone ME13.3) and APC-conjugated (eBioscience; clone 390) CD31, PE-Cy7-conjugated EpCAM (BioLegend, Inc.; clone 68.8), Pacific blue-conjugated CD45.2 (BioLegend, Inc.; clone 104), PerCP-Cy5.5-conjugated NK1.1 (BD Biosciences; clone PK136), PE-Cy7-conjugated CD3e (eBioscience; clone 145-2C11), e450-conjugated CD4 (eBioscience; clone L3T4), PE-conjugated CD8 (BD Biosciences; clone 53-6.1), Pacific blue-conjugated B220 (BD Biosciences; clone RA3-6B2), PE-conjugated CD19 (BD Biosciences, clone 1D3), PE-Cy7-conjugated CD11b (eBiosciences; clone M1/70), PerCP-Cy5.5-conjugated CD11c (eBiosciences; clone N418), APC-conjugated Gr-1 (BD Biosciences; clone RB6-8C5), Pacific blue- and PE-conjugated Ly6G (BD Biosciences; clone IA8), APC-conjugated F480 (eBioscience; clone BM8), PE-conjugated 7/4 (AbD Serotec; clone 7/4), PE-conjugated I/A-I/E (BD Biosciences; clone M5/114.15.2), PE-Cy7 conjugated CD205 (eBiosciences; clone 205yekta), Fitc-conjugated CD69 (BD Biosciences; clone H1.2F3) and APC-conjugated CD25 (eBiosciences; clone PC61.5). Flow cytometry acquisition was performed with BD FACSDiva™ -driven BD™ LSR II flow cytometer (Becton, Dickinson and Company). Data was then analyzed with FlowJo software (Treestar Inc.).
Western Blot
FACS purified lung cell populations (1 × 105 cells) using the antibodies described above were homogenized in RIPA buffer supplemented with protease inhibitors (Pierce). Lysates were centrifuges at 50,000×g for 30 minutes and the protein concentration in the supernatant were determined by BCA assay (Pierce). Equal amounts of protein from cell lystates were loaded in non-denaturing conditions and separated by SDS-PAGE in 4–12% NuPAGE (Novex) Bis-Tris gels. Gels were transferred to PVDF membranes followed by probing for GFP using an anti-GFP antibody (Abcam) and an anti-rabbit Ig light chain-HRP secondary (ELC Biosciences) using chemiluminescence autoradiography.
Supplementary Material
Highlights.
S1P1 signaling on pulmonary endothelium inhibits the initiation of cytokine storm
Cytokine production and leukocyte recruitment are independent events
S1P1 signaling regulates leukocyte recruitment by suppressing chemokine production by pulmonary endothelial cells
Cytokine production requires Type I Interferon signaling following virus infection
Blockade of cytokine amplification by S1P1 agonism enhances survival following
human pathogenic influenza virus infection
Acknowledgments
This is Publication Number 21112 from the Department of Immunology and Microbial Science and the Department of Chemical Physiology and The Scripps Research Institute Molecular Screening Center, The Scripps Research Institute (TSRI). This work was supported in part by USPHS grants AI074564 (MBAO, HR, KW, JT), AI009484 (MBAO), AI05509 (HR), MH084512 (HR) and NIH training grants NS041219 (KW), AI007244 (KW) and AI007364 (JT). We thank Marcus Boehm, Li-ming Huang and Bryan Clemons (Receptos, Inc) for helping provide RP-002 as a chemical tool.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergeron Y, Ouellet N, Deslauriers AM, Simard M, Olivier M, Bergeron MG. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramirez P, Martin-Loeches I, Varillas D, Gallegos MC, Seron C, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges AA, Campos GM, Moreli ML, Souza RL, Aquino VH, Saggioro FP, Figueiredo LT. Hantavirus cardiopulmonary syndrome: immune response and pathogenesis. Microbes Infect. 2006;8:2324–2330. doi: 10.1016/j.micinf.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Cahalan SM, Gonzalez-Cabrera PJ, Sarkisyan G, Nguyen N, Schaeffer MT, Huang L, Yeager A, Clemons B, Scott F, Rosen H. Actions of a picomolar short-acting S1P(1) agonist in S1P(1)-eGFP knock-in mice. Nat Chem Biol. 2011;7:254–256. doi: 10.1038/nchembio.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloniz C, Shinya K, Peng X, Korth MJ, Proll SC, Aicher LD, Carter VS, Chang JH, Kobasa D, Feldmann F, et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5:e1000604. doi: 10.1371/journal.ppat.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. Influenza virus receptor specificity: disease and transmission. Am J Pathol. 2010;176:1584–1585. doi: 10.2353/ajpath.2010.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, Schaeffer MT, Chapman J, Cameron M, Guerrero M, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol. 2008;74:1308–1318. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- Kawane K, Tanaka H, Kitahara Y, Shimaoka S, Nagata S. Cytokine-dependent but acquired immunity-independent arthritis caused by DNA escaped from degradation. Proc Natl Acad Sci U S A. 2010;107:19432–19437. doi: 10.1073/pnas.1010603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- Link H. The cytokine storm in multiple sclerosis. Mult Scler. 1998;4:12–15. doi: 10.1177/135245859800400104. [DOI] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Edelmann KH, Walsh KB, Guerrero M, Hatta Y, Kawaoka Y, Roberts E, Oldstone MB, Rosen H. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol. 2008;74:896–903. doi: 10.1124/mol.108.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone MB. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinborough E, Boehm M, Yeager A, Yamiyo J, Huang L, Brahmachary E, Moorjani M, Timony G, Brooks J, Peach R, et al. World International Patent Organization; WO/2011/060392. Selective sphingosine 1-phosphate receptor modulators and methods of chiral synthesis. 2011:92.
- Openshaw PJ, Dunning J. Influenza vaccination: lessons learned from the pandemic (H1N1) 2009 influenza outbreak. Mucosal Immunol. 2010;3:422–424. doi: 10.1038/mi.2010.34. [DOI] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera P, Marsolais D, Cahalan S, Don AS, Sanna MG. Modulating tone: the overture of S1P receptor immunotherapeutics. Immunol Rev. 2008;223:221–235. doi: 10.1111/j.1600-065X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- Rosen H, Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987;166:1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Liao J. Sphingosine 1-phosphate pathway therapeutics: a lipid ligand-receptor paradigm. Curr Opin Chem Biol. 2003;7:461–468. doi: 10.1016/s1367-5931(03)00085-1. [DOI] [PubMed] [Google Scholar]
- Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel V, Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine Growth Factor Rev. 2008;19:121–132. doi: 10.1016/j.cytogfr.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherne DM, Garcia-Sastre A. Virulence determinants of pandemic influenza viruses. J Clin Invest. 2011;121:6–13. doi: 10.1172/JCI44947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.