Abstract
With yeast two-hybrid methods, we used a C-terminal fragment (residues 1697-2145) of non-erythroid beta spectrin (βII-C), including the region involved in the association with alpha spectrin to form tetramers, as the bait to screen a human brain cDNA library to identify proteins interacting with βII-C. We applied stringent selection steps to eliminate false positives and identified 17 proteins that interacted with βII-C (IPβII-C s). The proteins include a fragment (residues 38-284) of “THAP domain containing, apoptosis associated protein 3, isoform CRA g”, “glioma tumor suppressor candidate region gene 2” (residues 1-478), a fragment (residues 74-442) of septin 8 isoform c, a fragment (residues 704-953) of “coatomer protein complex, subunit beta 1, a fragment (residues 146-614) of zinc-finger protein 251, and a fragment (residues 284-435) of syntaxin binding protein 1. We used yeast three-hybrid system to determine the effects of these βII-C interacting proteins as well as of 7 proteins previously identified to interact with the tetramerization region of non-erythroid alpha spectrin (IPαII-N s) [1] on spectrin tetramer formation. The results showed that 3 IPβII-C s were able to bind βII-C even in the presence of αII-N, and 4 IPαII-N s were able to bind αII-N in the presence of βII-C. We also found that the syntaxin binding protein 1 fragment abolished αII-N and βII-C interaction, suggesting that this protein may inhibit or regulate non-erythroid spectrin tetramer formation.
Keywords: Brain beta spectrin, spectrin tetramerization, brain proteins, yeast three-hybrid, library screening
INTRODUCTION
Spectrin is a cytoskeletal protein, initially identified for its role in preserving the biconcave shape of erythrocyte membranes [2] and originally considered to be present only in erythrocyte [3] until the identification of non-erythrocyte isoforms and their functions [4]. Spectrin is involved in the formation and maintenance of plasma membranes at sites of cell-cell contacts [5], protein sorting and accumulation [6], interactions with structural and regulatory proteins [7], regulation of signal transduction pathways [8], and regulation of DNA repair [9]. Non-erythroid spectrin (spectrin II), also referred to as brain spectrin [10], calspectin [11], or fodrin [12], is found in neuronal axons [13], whereas erythroid spectrin (spectrin I) is confined to neuronal cell bodies and dendrites, and some glial cells [14]. Beta II spectrin (βII) is found in cell bodies as well as axons (e.g. [15]). Furthermore, βI spectrin is absent from Purkinje cell dendrites [16]. βII participates in the propagation of TGF-β signaling [17]. Gene knock-out studies show that spectrin expression and regulation are important for fundamental cellular functions. Many spectrin mutations are non-lethal but cause disease conditions in humans [18]. Studies have also shown that knockdown of αII spectrin is lethal, and spectrin is an essential protein in the cell [5, 19, 20]. Spectrin tetramer is the functional form in many cells [21, 22], and, for example, spectrin tetramer is important in neuritogenesis [22].
Spectrin tetramerization involves interaction of the lone helix (Helix C’) at the N-terminal region of α-spectrin of one αβ heterodimer and the two helices (Helix A’ and Helix B’) at the C-terminal region of the β-spectrin on another heterodimer [23 - 26]. This interaction involves hydrophobic residue clustering, salt bridges and hydrogen bonds [25 - 29]. Despite high sequence homology and three-dimensional structural similarity, dissociation constant measurements using model proteins of different spectrin fragments show two orders of magnitude difference in the N-terminal α-spectrin and C-terminal β-spectrin association affinity between erythroid and non-erythroid spectrin [30, 31], in good agreement with earlier studies using intact spectrin [32]. It has been shown that other proteins also interact with the N-terminal region of αII-spectrin [1]. They include Duo protein, Lysyl-tRNA synthetase, TBP associated factor 1, two isoforms (b and c) of a protein kinase A interacting protein and 2 different segments of Zinc finger protein 333 as well as several unknown proteins. These proteins may compete with its spectrin partner to regulate spectrin tetramerization and cytoskeletal structures.
In this study, we identified seventeen proteins that interact with a recombinant protein consisting of the C-terminal tetramerization site of βII-spectrin (βII-C). The proteins include a fragment (residues 38-284) of “THAP domain containing, apoptosis associated protein 3, isoform CRA g”, “glioma tumor suppressor candidate region gene 2”, a fragment (residues 74-442) of septin 8 isoform c, a fragment (residues 704-953) of “coatomer protein complex, subunit beta 1”, a fragment (residues 146-614) of zinc-finger protein 251, and a fragment (residues 284-435) of syntaxin binding protein 1. These 17 proteins, along with 7 proteins that interact with the N-terminal region of αII-spectrin (αII-N) mentioned above, have been tested for their effects on spectrin tetramerization. One βII-C interacting protein abolishes αII-N and βII-C interaction. This protein of 153 residues, except the last 8 residues, is identical to a fragment (residues 284-428) of syntaxin binding protein 1. We also studied the effects of these proteins on αII-N and βII-C association and found that the binding of syntaxin binding protein 1 fragment to βII-C abolishes the αII-N and βII-C association, suggesting that this protein may inhibit or regulate non-erythroid spectrin tetramerization.
MATERIALS AND METHODS
Library Screening for βII-C Interacting Proteins (IPβII-C)
The C-terminal region (amino acid residues 1697-2145) of brain (non-erythroid) beta spectrin (accession number Q01082) (βII-C) was used as the bait to screen for interacting proteins in the human brain cDNA library (BD Matchmaker Library, BD Biosciences Clontech). The sequence encoding βII-C was cloned to the binding domain (BD) plasmid (pBD) using standard methods [1, 33], and labeled as pBD-βII-C.
To test for potential toxic effects of BD-βII-C fusion protein, AH109 cells were co-transformed with pBD-βII-C and an empty activation domain (AD) plasmid (pAD). Briefly, several colonies of AH109 cells were grown in medium with yeast extract, peptone, dextrose, and adenine (YPDA, 50 mL) at 30 °C overnight, before transferring to a fresh YPDA with kanamycin (300 mL) until an OD600 of 0.6 (about 3 hours), following procedures in the user manual. Cells were harvested, washed with tris-EDTA solution and suspended in tris-EDTA plus lithium acetate solution (see user manual for solution preparation). pBD-βII-C and pAD plasmids (0.1 μg of each), and Herring Testes carrier DNA (0.1 mg) were mixed with the cell suspension (100 μL). Polyethylene glycol and lithium acetate solution (600 μL) was added and the mixture was incubated at 30 °C for 30 min. Dimethyl sulfoxide (70 μL) was added, before a heat shock step at 42 °C for 15 min. Cells were briefly centrifuged, re-suspended in sterile tris-EDTA solution before spreading on agar plates containing synthetic defined (SD) minimal medium with double drop-out (DDO, SD/-Leu/-Trp) supplement and grown for 3 days at 30 °C.
To test for potential non-specific activation of the reporter genes giving false positive results in screening, AH109 cells with pBD-βII-C and an empty pAD plasmids were spread on agar plates containing SD minimal medium supplemented with quadruple drop-out (QDO, SD/-Ade/-His/-Leu/-Trp) and grown for 3 days at 30 °C.
For library screening, bait plasmid pBD-βII-C was transformed into yeast strain AH109. A freshly transformed colony, 2-3 mm in size, was inoculated into SD medium with drop-out supplement lacking tryptophane (SD/-Trp, 50 mL) and grown until cells reached stationary phase (OD600 > 1.5). AH109 cells were harvested, re-suspended with a “2X YPDA” plus kanamycin solution (5 mL) and mated with Y187 cells containing library plasmids (pAD-IPβII-C) with > 5 × 107 cfu/mL (1 mL). These cells were cultured again in 2X YPDA with kanamycin (45 mL) for 20 hours at 30 °C with slow shaking (30-50 rpm). Diploid cells were collected and spread on 50 large (150 mm) plates containing SD medium with QDO supplement and grown for 5 days at 30 °C. Well isolated colonies growing on these plates were selected, and ones showing coalescent growth were avoided.
Further selection to obtain positive colonies was done by transferring selected colonies to QDO plates with the chromagenic substance, X-α-gal, and grown for 3 days at 30 °C. Those colonies with α-galactosidase production were detected by the appearance of blue colonies as they grew on plates.
Co-transformation for Confirmation of Screened Interacting Proteins
Plasmids purified from positive colonies were transformed into E. coli DH5α cells, using conventional methods. Cells that were able to grow on plates with ampicillin were used to eliminate kanamycin resistant pBD-βII-C plasmid, and to obtain pAD-IPβII-C plasmids in positive colonies. Purified pAD-IPβII-C and pBD-βII-C plasmids were co-transformed into the AH109 cells and plated on QDO plates. After 3 days at 30 °C, cells without growth were eliminated, and only those with growth were further analyzed for IPβII-C s.
Effects of Interacting Proteins on Spectrin Tetramerization
We also identified the IPβII-C s that were able to bind to βII-C in the presence of αII-N (first 359 residues in αII) by using the yeast three-hybrid vector, pBridge (pBR), to express not only the binding domain fusion protein, BD-βII-C, but also to express an additional protein (such as αII-N) only in the absence of methionine in the growth medium. In the presence of methionine, this additional protein was not expressed, and thus can be used as a control sample. AH109 cells were co-transformed with pBR-βII-C--αII-N and pAD-IPβII-C plasmids. These cells were plated on agar plates containing SD medium with TDO supplement in the absence of methionine in the growth medium to express BD-βII-C and AD-IPβII-C as well as αII-N, and in the presence of methionine to express only BD-βII-C and AD-IPβII-C, and allowed to grow for 3 days at 30 °C.
Once the IPβII-C s that were able to bind to βII-C in the presence of αII-N were selected, we then selected those that abolish the interaction between βII-C and αII-N by using AH109 cells co-transformed with pBR-αII-N--IPβII-C and pAD-βII-C plasmids to express BD-αII-N and AD-βII-C as well as IPβII-C, in the absence of methionine.
In addition to IPβII-C s from this screening, we also studied the effect of αII-N interacting proteins (IPαII-N s) identified in our earlier screening [1], on αII-N and βII-C interaction by using a similar experimental set up, replacing IPβII-C with IPαII-N in the plasmids used. The seven proteins used were Zinc finger protein 333 - fragment 1-169, Zinc finger protein 333 - fragment 1-230, AKIP1b, lysyl-tRNA synthetase - fragment 1-151, TBP associated factor 1-fragment 1270-1495, Duo protein - fragment 181-722 and spectrin βIV - fragment 1916-2564 [1].
DNA sequencing and protein identification
Plasmids from positive colonies with IPβII-C s were sequenced at the DNA Services Facility, Research Resources Center at the University of Illinois at Chicago. Sequencing results were analyzed with Clustal W v1.7 (EMBL, Heidelberg, DE) to identify the sequences of SMART III, CDSIII, and poly A tail in each plasmid, and the segment between the SMARTIII and CDSIII sequences was marked as the sequence for the library cDNA. Since the sequence of SMARTIII may vary in pAD-IPβII-C plasmids (see Clontech manual for SMART cDNA library construction), all three possible reading frames of DNA sequence were examined (Frame 0, following the Clontech codon assignment for SMARTIII for the rest of the plasmid; Frame +1, frame with one additional nucleotide; Frame −1, frame with two additional nucleotides). The frame containing the most codons before the first stop codon was selected. Amino acid sequences were determined using the Translate tool (ExPASy proteomics server), and the sequences between SMARTIII and the first stop codon was taken as the sequence for the interacting protein. These sequences were analyzed using Blastn, Blastx, and Blastp in “Basic Local Alignment Tool” (http://www.ncbi.nlm.nih.gov/blast/) in all non-redundant BLAST protein sequence databases, as before [1], for information on the interacting proteins.
RESULTS
Library Screening for βII-C Interacting Proteins
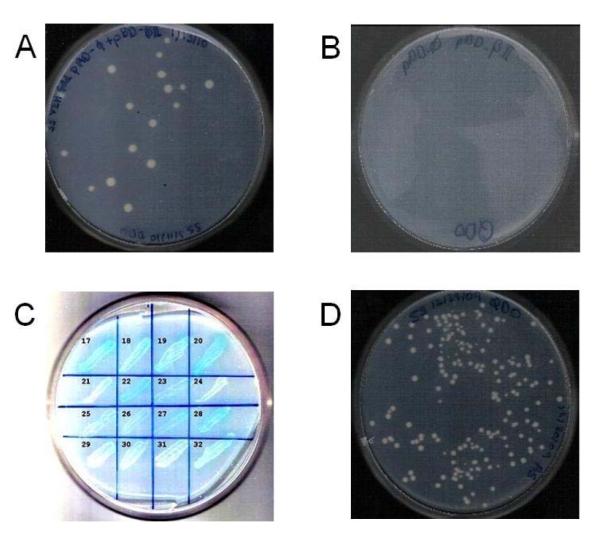
Tests for toxic effects of βII-C on yeast growth showed several colonies, 2-5 mm in diameter (Figure 1A), indicating that βII-C is not toxic to the yeast cells. In the test for false positive in screening, yeast cells with pBD-βII-C and empty pAD plasmids did not form any colonies (Figure 1B), indicating that colony growth is observed only in the presence of an interacting protein X expressed by the pAD-X plasmid.
Figure 1. Library Screening for βII-C Interacting Proteins.
AH109 cell colonies with pBD-βII-C and pAD plasmids supplemented with Ade and His (DDO medium) after 3 days of growth at 30 °C, indicating that pBD-βII-C is not toxic to yeast cell growth (A). Same cells without Ade and His supplement (QDO medium) after 3 days of growth at 30 °C, shows no colonies indicating that pBD-βII-C does not lead to the false activation of the reporter genes (B). Colonies from screening transferred to QDO plates with X-α-gal, and grown for 3 days at 30 °C, where 59 colonies, such as on grids 17-20, 22-23, 25-28 and 32, turned blue, while most of the colonies, such as on grids 21, 24 and 29-31, grew as white colonies (C). AH109 cells with one of the 20 randomly selected sequences (pAD-IPβII-C-1) and pBD-βII-C plasmids on QDO plates, after 3 days of growth at 30 °C, shows high numbers of colonies, confirming the presence of positive interactions between βII-C and IPβII-C-1 (D). In the library screening experiments for identifying βII-C interacting proteins, we selected 299 well separated colonies and avoided coalescent colonies. We further selected only those colonies (a total of 59) that produced α-galactosidase to give blue colonies (Figure 1C, for example, colonies on grids 17-20, 22-23, 25-28 and 32) and eliminated 240 of those colonies that appeared as white colonies (Figure 1C, colonies on grids 21, 24 and 29-31) and were considered to be false positives. In the co-transformation confirmation analysis, randomly selected 20 of the 59 blue colonies showed colony growth in all 20 samples (Figure 1D), confirming that these colonies indeed consisted of proteins that interacted with βII-C.
DNA Sequencing Results of the βII-C Interacting Proteins
Sequence analysis of the cDNA sequences between SMARTIII and CDSIII of the 20 confirmed plasmids revealed that the sequences of the cDNA fragments ranged from 487 to 1,744 nucleotides. Three of the cDNA sequences were identical to each other, and another 2 of the cDNA sequences were also identical to each other (Table 1). Thus, a total of 17 different sequences were obtained from the 20 randomly selected positive colonies (Table 1). Selecting the largest number of codons from one of the three frames (−1, 0 and +1 frames, see Methods), 8 of the 17 sequences were with frame 0, with the SMARTIII sequence ending as ATG GCC (Table 1, #1-5 and 7-9). For #6 only 49 codons were obtained for frame 0, but 303 codons for frame +1, suggesting that the SMARTIII sequence for this sample ended with one extra nucleotide (G in TTA TGG CCG). A similar frame shift was observed for #11 and 17.
Table 1.
DNA sequencing analysis of the library plasmids that show positive interactions with the C-terminal region (residues 1697-2145) of non-erythroid β spectrin (βII-C).
| IPβII-C | Nucleotidesa | Frameb | Codonsc | First Three Residuesd | Occurrence |
|---|---|---|---|---|---|
| 1 | 1536 | 0 | 153 (6, 30) | DDD | 3 |
| 2 | 1509 | 0 | 483 (0, 4) | SSF | 1 |
| 3 | 1509 | 0 | 483 (0, 4) | SSF | 1 |
| 4 | 1744 | 0 | 278 (10, 39) | RVG | 1 |
| 5 | 1325 | 0 | 386 (10, 39) | RVG | 1 |
| 6 | 1400 | 1 | 303 (7, 49) | KKK | 2 |
| 7 | 1232 | 0 | 247 (42, 108) | GGS | 1 |
| 8 | 602 | 0 | 131 (16, 24) | GGR | 1 |
| 9 | 1026 | 0 | 250 (5, 8) | EAA | 1 |
| 10 | 784 | −1 | 260 (21, 30) | ELG | 1 |
| 11 | 1678 | 1 | 124 (73, 40) | LGK | 1 |
| 12 | 1484 | −1 | 369 (4, 26) | ASH | 1 |
| 13 | 487 | −1 | 54 (16, 3) | GEV | 1 |
| 14 | 1121 | −1 | 84 (14, 28) | PQP | 1 |
| 15 | 1083 | −1 | 26 (7, 15) | ERE | 1 |
| 16 | 889 | −1 | 18 (10, 3) | QAW | 1 |
| 17 | 1672 | 1 | 78 (39, 27) | ILP | 1 |
Number of nucleotides between SMARTIII and CDSIII;
Frame 0 uses the codon assignment by Clontech for SMARTIII sequence; Frame +1 is with one extra nucleotide; Frame −1 is with two extra nucleotides;
Number of codons for the assigned frame, with those for other frames given in parentheses and the numbers for Frame 0 bolded;
first three amino acid residues in the protein.
For #10, only 21 codons were obtained for frame 0, 30 codons for frame +1, but 260 codons for frame −1, suggesting that the SMARTIII sequence for this sample ends with two extra nucleotides (GG in TAT GGC CGG). Similar frame shift was observed for #12-16. The first three amino acid residues of each translated proteins are shown in Table 1 for identification references.
αII-N Effect on IPβII-C Interaction with βII-C
The yeast three-hybrid experiments with the cells of the 17 samples grown in the presence of methionine to express AD-IPβII-C and BD-βII-C, but not αII-N show colony growth (data not shown), as expected, confirming the interactions between AD-IPβII-C and BD-βII-C in these cells. However, for cells grown in the absence of methionine leading to the expression of αII-N alongside AD-IPβII-C and BD-βII-C, 14 samples showed no colony formation, indicating these IPβII-C s did not interact with βII-C in the presence of αII-N. Only the cells with three IPβII-C s (IPβII-C-1, -8 and -9) gave colonies (Table 2A), indicating that IPβII-C-1, -8 and -9 interacted with βII-C in the presence of αII-N. Of these three IPβII-C s that interact with βII-C in the presence of αII-N, we expressed IPβII-C-1 in cells with BD-αII-N and AD-βII-C (pBR-αII-N--IPβII-C and pAD-βII-C plasmids, in the absence of methionine) and found no colony growth (Table 2C), indicating that the presence of IPβII-C-1 abolished the αII-N and βII-C interaction. In the presence of methionine, when no IPβII-C -1 was expressed, colonies formed (data not shown).
Table 2.
Results of Yeast Three-Hybrid Experiments for (A) Effects of αII-N on βII-C and IPβII-C Interaction (B) Effects of βII-C on αII-N and IPαII-N Interaction, and (C) Effect of IPβII-C on αII-N and βII-C Interaction.
| A. Cells with plasmids pAD-IPβII-C and pBR-βII-C--αII-N were grown in the absence of methionine to express AD-IPβII-C, BD-βII-C and αII-N. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IPβII-C | IPβII-C-1 | IPβII-C-2 | IPβII-C-3 | IPβII-C-4 | IPβII-C-5 | IPβII-C-6 | IPβII-C-7 | IPβII-C-8 | IPβII-C-9 |
|
Colony
Growth |
yes | no | no | no | no | no | no | yes | yes |
Alignment of βII-C Interacting Protein Sequences to Human Proteins
The sequence alignment results of the 17 IPβII-C sequences to protein sequences in the database show that only IPβII-C-7, except the last 8 residues, is identical to a fragment (residues 38-284) of a known protein -- “THAP domain containing, apoptosis associated protein 3, isoform CRA g” (Table 3). Four of the proteins were 99% identical to known proteins or protein fragments, including glioma tumor suppressor candidate region gene 2 (residues 1-478), septin 8 isoform c (residues 74-442), and coatomer protein complex, subunit beta 1 (residues 704-953) (Table 3). We are puzzled about the identity of IPβII-C-10, which is identical to βII spectrin, residues 1781-2040 except with two mutations (Table 3). IPβII-C-4 was similar to zinc-finger protein 251 (residues 146-614) (2% difference), and IPβII-C-1 was similar to syntaxin binding protein 1 (residues 284-435) (4% difference). The remaining proteins exhibited lower homology values to known proteins (Table 3).
Table 3.
βII-C Interacting Proteins (IPβII-C s) and Their Effects on Tetramerization Site Interaction
| IPβII-C | Matching Sequence |
Homo sapiens Proteins in Databases (Accession #), Matching Fragment |
Difference | αII-Na | Effect on Tetramerb |
|---|---|---|---|---|---|
| 7 | 1-247 (247)c | THAP domain containing, apoptosis associated protein 3, isoform CRA g (EAW71568), residues 38-284 (284)c |
0% | yes | no |
| 10 | 1-260*d (260) | Spectrin, beta, non-erythroid (Q01082), residues 1781-2040 (2314) *G20E, F235Sd |
1% | yes | no |
| 2 | 6-483 (483) | Glioma tumor suppressor candidate region gene 2 (NP_056525), residues 1-478 (478) |
1% first 5 aa (SSFDK) |
yes | no |
| 12 | 1-369* (369) | Septin 8 isoform c (NP_001092282), residues 74-442 (442) | 1% *H239Q, P344T, F362S |
yes | no |
| 9 | 1-250* (250) | Coatomer protein complex, subunit beta 1 (NP_057535), residues 704-953 (953) |
1% *Y53C |
no | not tested |
| 4 | 1-271 (278) | Zinc-finger protein 251 (NP_612376), residues 146-416 (671) | 2% last 7 residues |
yes | no |
| 1 | 1-145 (153) | Syntaxin binding protein 1 (NP_003156), residues 284-428 (603) |
4% last 8 residues |
no | yes |
| 3 | 6-263, 288-483 (483) |
Glioma tumor suppressor candidate region gene 2 (NP_056525), residues 1-258; 283-478 (478) |
6% first 5 aa (SSFDK) |
yes | no |
| 8 | 19-131 (131) | Ubiquitin-conjugating enzyme E2L3 (BAG61806), residues 100-212 (212) |
14% first 18 aa |
no | not tested |
| 6 | 1-247*e (303) | Golgin A6 family-like 10 (NP_001157937), (479) | 26% | yes | no |
| 5 | 32-386* (386) | Zinc-finger protein 251 (NP_612376), residues 317-671 (671) | 27% (*numerous mutations) |
yes | no |
| 11 | 4-79 (124) | Eukaryotic translation initiation factor 3, subunit H (EAW91959), residues 1-76 (332) |
30% (*numerous mutations) |
yes | no |
| 13-17 | Unknowns 1, 2, 3, 4 | yes | no |
effects of αII-N on IPβII-C and βII-C interaction, with “yes” indicating that αII-N abolishes IPβII-C interaction with βII-C;
effects of IPβII-C on αII-N and βII-C interaction;
total number of residues in protein;
the symbol * indicates mutations exist.
IPβII-C res 1-150 correspond to res 47-196 with 5 mutations (I4V, E39Q, R156H, R163C, Q169L, D174E, R191C), res 151-168 correspond to res 211-228 and res 169-247 correspond to res 243-321 with E237D.
Those proteins identified that deviate from the human prototype standards, even by a single amino acid, should be considered as a “new” protein. The mutation(s) may or may not affect the interactions with the bait protein. For example, IPβII-C-4 was similar to zinc-finger protein 251 fragment (residues 146-614), but with 2% difference. Our results provide no information on whether zinc-finger protein 251 fragment (residues 146-614) interacts with βII-C, unless verified by additional analysis using the fragment of zinc-finger protein 251 (residues 146-614). Thus, some of the interactions reported here may represent artifactual positives based on mutant sequences.
βII-C Effect on IPαII-N Interaction with αII-N
For the 7 αII-N interactors identified previously (see Methods), cells grown in the presence of methionine, consisting of AD-IPαII-N and BD-αII-N, but no βII-C, showed colony growth, confirming the interactions between AD-IPαII-N and BD-αII-N in these cells (data not shown). Four of the 7 samples of cells grown in the absence of methionine, with βII-C being expressed along side with AD-IPαII-N and BD-αII-N, gave colonies, indicating that IPαII-N-1 (TBP-associated factor), IPαII-N-2 (lysyl-tRNA synthetase), and IPαII-N-4 and IPαII-N-5 (two fragments of Zinc finger protein 333, 1-169 residues and 1-230 residues fragments, respectively) interacted with αII-N in the presence of βII-C. Cells with IPαII-N-3, IPαII-N-6 and IPαII-N-7 did not show any colony growth (Table 2B).
Of the 4 IPαII-N s that interact with βII-C in the presence of αII-N, Zinc finger protein 333 - fragment 1-230 was selected to test for its effect on αII-N and βII-C interactions. In the absence of methionine, with the presence of Zinc finger protein 333 - fragment 1-230, colony growth was observed, indicating that Zinc finger protein 333 - fragment 1-230 did not abolish the αII-N and βII-C interaction (Table 2C).
DISCUSSION
Tetramerization is an important process for spectrin isoforms, and involves helical bundling of three helices, one from the α- and two from the β-spectrin [14, 25, 28]. The bundled complexes exhibit different Kd values, with the non-erythroid complex αII-N/βII-C about 10 nM and the erythroid complex about 1 μM [31]. Proteins have been identified that interact with αII spectrin at the tetramerization site, and we suggest that these proteins may regulate the affinity between αII-N and βII-C [1]. In this study, we identified 17 proteins that interacted with βII spectrin at the tetramerization site. Eight of these 17 proteins were very similar to existing proteins, with one (IPβII-C-7) identical to “THAP domain containing, apoptosis associated protein 3, isoform CRA g”. Each member of the THAP family consists of a conserved domain [34], the THAP domain, which is a putative DNA-binding domain and probably also binds a zinc ion. This is a novel protein motif with similarity to the DNA-binding domain of P element transposase in Drosophila [35]. Another βII-C interactor (IPβII-C-4) also binds zinc ion and is identical to a fragment (residues 146-416) of zinc finger protein 251 (ID#: Q9BRH9), with an additional 7 residues at the C-terminus. It is interesting to note that fragments (residues 1-169 and 1-230) of zinc finger protein 333 are αII-N interacting proteins [1]. And, in this study, we found that these fragments associate with αII-N even in the presence of βII-C, but it did not abolish the αII-N and βII-C association. Similarly, the zinc finger protein 251 fragment associates with βII-C even in the presence of αII-N, but it did not abolish the αII-N and βII-C association in tetramer formation. IPβII-C-5 is also similar to zinc finger protein 251 (residues 317-671), but with numerous mutations. IPβII-C-2, other than the first 5 residues, is identical to “glioma tumor suppressor candidate region gene 2”, in its entirety. It is also interesting that we identified another protein (IPβII-C-3) that consists of the first 258 residues and residues 283-478 of this protein. IPβII-C-12 is identical to a C-terminal fragment (residues 74-442) of septin 8 isoform c, except with three mutations (H239Q, P344T and F362S). Septin 8 isoform c is a member of the large septin family that performs diverse cellular functions according to tissue expression and their interacting partners. Functions include cell division, chromosome segregation, protein scaffolding, cellular polarity, motility, membrane dynamics, vesicle trafficking, exocytosis, apoptosis, and DNA damage response [36, 37]. The 6 IPβII-C s discussed above did not interact with βII-C in the presence of αII-C, suggesting that their affinities with βII-C are weaker than that of αII-N with βII-C.
IPβII-C-9, identical to the C-terminal 250 residues of coatomer subunit beta (residues 704-953) except with one mutation (Y to C, residue Y756 in coatomer subunit beta and residue C53 in IPβII-C-9), interacts with βII-C even in the presence of αII-N, suggesting strong affinity with βII-C. Similar to IPβII-C-9 in affinity are IPβII-C-1 and IPβII-C-8. IPβII-C-1, except for the last 8 residues, is identical to a fragment (residues 284-428) of syntaxin binding protein 1. Syntaxin binding protein 1 appears to play a role in the release of neurotransmitters via regulation of syntaxin, a transmembrane attachment protein receptor [38]. IPβII-C-8, except the first 18 residues, is identical to ubiquitin-conjugating enzyme E2L3, residues 100-212. This enzyme participates in the ubiquitination of p53, c-Fos and the NF-κB precursor p105 in vitro [39, 40]. With these three strongly interacting proteins, only IPβII-C-1, a 153-residue protein and its residues 1-145, which are identical to residues 284-428 of syntaxin binding protein 1, abolished αII-N and βII-C interaction. Until demonstrated by future experimental results, it is also possible that the interactions between specific IPβII-C and βII-spectrin may not regulate spectrin tetramer formation. It is possible that these interactions may play a role in other cellular processes. As indicated in a recent review, the spatial and temporal organization of molecules within a cell is critical for coordinating the many distinct activities carried out by the cell [41]. Scaffold proteins, including actin-spectrin cytoskeleton, have been found to play a central role in physically assembling the relevant molecular components, and have been exploited by evolution, pathogens, and cellular engineers to reshape cellular behavior. The IPβII-C s identified in this work may play a role in some of these cellular activities.
In summary, we have identified 17 human proteins or protein fragments that interact with βII-C, a region of the non-erythroid beta spectrin that is involved in spectrin tetramerization. Most of these proteins (14 of them) appear to interact with βII-C with lower affinity than that of αII-N since they do not interact with βII-C in the presence of αII-N. However, three of these proteins retain interactions with βII-C in the presence of αII-N, and one, the syntaxin binding protein fragment, abolishes αII-N and βII-C interactions. We suggest that further studies of these interactions, on structural and cellular levels, will provide a better understanding of brain physiology and pathophysiology.
| IPβII-C | IPβII-C-10 | IPβII-C-11 | IPβII-C-12 | IPβII-C-13 | IPβII-C-14 | IPβII-C-15 | IPβII-C-16 | IPβII-C-17 |
|---|---|---|---|---|---|---|---|---|
|
Colony
Growth |
no | no | no | no | no | no | no | no |
| B. Cells with plasmids pAD-IPαII-N, and pBR-αII-N--βII-C were grown in the absence of methionine to express AD-IPαII-N, BD-αII-N and βII-C. | |||||||
|---|---|---|---|---|---|---|---|
| IPαII-N | IPαII-N-1 | IPαII-N-2 | IPαII-N-3 | IPαII-N-4 | IPαII-N-5 | IPαII-N-6 | IPαII-N-7 |
|
Colony
Growth |
yes | yes | no | yes | yes | no | no |
| C. Cells with plasmids pAD-βII-C and pBR-αII-N--IPβII-C-1, or pBR-αII-N--IPαII-N-5, were grown in the absence of methionine to express AD-βII-C, BD-αII-N and IPβII-C-1 or IPαII-N-5. | ||
|---|---|---|
| IP | IPβII-C-1 | IPαII-N-5 |
|
Colony
Growth |
no | yes |
List of the abbreviations
- αII
non-erythroid (brain) alpha spectrin
- αII-N
a recombinant protein consisting of the N-terminal region 359 residues of αII
- AD
activation domain of GAL4
- βII
non-erythroid (brain) beta spectrin
- βII-C
a recombinant protein consisting of residues 1697-2145 at the C-terminus of βII
- BD
binding domain of GAL4
- IPαII-N
proteins interacting with αII-N
- IPβII-C
proteins interacting with βII-C
- pAD
yeast two-hybrid cloning vector pGADT7
- pBD
yeast two-hybrid cloning vector pGBKT7
- pBR
yeast three-hybrid cloning vector pBridge
- QDO
quadruple drop-out
- SD
synthetic defined
- TDO
triple drop-out
- X-α-gal
5-bromo-4-chloro-3-indolyl-α-galacto-pyranoside
- YPDA
yeast growth medium with yeast extract, peptone, dextrose and adenine
REFERENCES
- 1.Oh Y, Fung LW-M. Brain proteins interacting with the tetramerization region of non-erythroid alpha spectrin. Cell. Mol. Biol. Lett. 2007;12:604–620. doi: 10.2478/s11658-007-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi VT, Steers E. Selective solubilization of a protein component of the red cell membrane. Science. 1968;159:203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- 3.Hiller G, Weber K. Spectrin is absent in various tissue culture cells. Nature. 1977;299:181–183. doi: 10.1038/266181a0. [DOI] [PubMed] [Google Scholar]
- 4.Levine J, Willard M. Axonally transported polypeptides associated with the internal periphery of many cells. J. Cell Biol. 1981;90:631–643. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JK, Coyne RS, Dubreuil RR, Goldstein LSB, Branton D. Cell shape and interaction defects in α-spectrin mutants of Drosophila Melanogaster. J. Cell Biol. 1993;123:1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinder JC, Baines AJ. A protein accumulator. Nature. 2000;406:253–254. doi: 10.1038/35018679. [DOI] [PubMed] [Google Scholar]
- 7.Djinovic-Carugo K, Gautel M, Ylanne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002;513:119–123. doi: 10.1016/s0014-5793(01)03304-x. [DOI] [PubMed] [Google Scholar]
- 8.Gascard P, Mohandas N. New insights into functions of erythroid proteins in nonerythroid cells. Curr. Opin. Hematol. 2000;7:123–129. doi: 10.1097/00062752-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Sridharan DM, McMahon LW, Lambert MW. II-spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol. Int. 2006;30:866–878. doi: 10.1016/j.cellbi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Kanda K, Tanaka T, Sobue K. Calspectin (fodrin or nonerythroid spectrin)-actin interaction: a possible involvement of 4,1-related protein. Biochem. Biophys. Res. Commun. 1986;140:1051–1058. doi: 10.1016/0006-291x(86)90741-2. [DOI] [PubMed] [Google Scholar]
- 11.Tsukita S, Tsukita S, Ishikawa H, Kurokawa M, Morimoto K, Sobue K, Kakiuchi S. Binding sited of calmodulin and actin on the brain spectrin, calspectin. J. Cell Biol. 1983;97:574–578. doi: 10.1083/jcb.97.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobue K, Kanda K, Kakiuchi S. Solubilization and partial purification of protein kinase systems from brain membranes that phosphorylate calspectin: a spectrin-like calmodulin-binding protein (fodrin) FEBS Lett. 1982;150:185–190. doi: 10.1016/0014-5793(82)81331-8. [DOI] [PubMed] [Google Scholar]
- 13.Riederer BM, Lopresti LL, Krebs KE, Zagon IS, Goodman SR. Brain spectrin (240/235) and brain spectrin (240/235E): conservation of structure and location within mammalian neural tissue. Brain Res. Bull. 1988;21:607–616. doi: 10.1016/0361-9230(88)90200-6. [DOI] [PubMed] [Google Scholar]
- 14.Ohara O, Ohara R, Yamakawa H, Nakajima D, Nakayama M. Characterization of a new β-spectrin gene which is predominantly expressed in brain. Mol. Brain Res. 1998;57:181–192. doi: 10.1016/s0169-328x(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Katuri V, Iqbal S, Narayan T, Wang Z, Lu RS, Mishra L, Mishra B. Elf a beta-spectrin is a neuronal precursor cell marker in developing mammalian brain; structure and organization of the elf/beta-g spectrin gene. Oncogene. 2002;21:5255–5267. doi: 10.1038/sj.onc.1205548. [DOI] [PubMed] [Google Scholar]
- 16.Lambert S, Bennett V. Postmitotic expression of ankyrinr and beta r-spectrin in discrete neuronal populations of the rat brain. J. Neurosci. 1993;13:3725–3735. doi: 10.1523/JNEUROSCI.13-09-03725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Y, Katuri V, Dillner A, Mishra B, Deng C-X, Mishra L. Disruption of transforming growth factor-β signaling in ELF β-spectrin-deficient mice. Science. 2003;299:574–577. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 18.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 19.Norman KR, Moerman DG. Alpha spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegant. J. Cell. Biol. 2002;157:665–677. doi: 10.1083/jcb.200111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon KR, Zhang P, Sridharan DM, Lefferts JA, Lambert MW. Knockdown of alpha II spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem. Biophys. Res. Commun. 2009;381:288–293. doi: 10.1016/j.bbrc.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSilva TM, Peng K-C, Speicher KD, Speicher DW. Analysis of human red cell spectrin tetramer (head-to-head) assembly using complementary univalent peptides. Biochemistry. 1992;31:10872–10878. doi: 10.1021/bi00159a030. [DOI] [PubMed] [Google Scholar]
- 22.Bignone PA, King MD, Pinder JC, Baines AJ. Phosphorylation of a threonine unique to the short C-terminal isoform of betaII-spectrin links regulation of alpha-spectrin interaction to neuritogenesis. J. Biol. Chem. 2007;232:888–896. doi: 10.1074/jbc.M605920200. [DOI] [PubMed] [Google Scholar]
- 23.Speicher D, DeSilva T, Speicher K, Ursitti J, Hembach P, Weglarz L. Location of the human red cell spectrin tetramer binding site and detection of a related “closed” hairpin loop dimer using proteolytic footprinting. J. Biol. Chem. 1993;268:4227–4235. [PubMed] [Google Scholar]
- 24.Mehboob S, Luo B-H, Fu W, Johnson ME, Fung LW-M. Conformational studies of the tetramerization site of human erythroid spectrin by cysteine-scanning spin-labeling EPR methods. Biochemistry. 2005;44:15898–15905. doi: 10.1021/bi051009m. [DOI] [PubMed] [Google Scholar]
- 25.Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragon A, Speicher DW. Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood. 2010;115:4843–4852. doi: 10.1182/blood-2010-01-261396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Y, Antoniou C, Memic A, Kay BK, Fung LW-M. Apparent structural differences at the tetramerization region of erythroid and nonerythroid beta spectrin as discriminated by phage displayed scFvs. Protein Sci. 2011;20:867–879. doi: 10.1002/pro.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antoniou A, Lam VQ, Fung LW-M. Conformational changes at the tetramerization site of erythroid α-spectrin upon binding β-spectrin: a spin label EPR study. Biochemistry. 2008;47:10765–10772. doi: 10.1021/bi800840p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y, Pipala NH, Fung LW-M. The L49F mutation in alpha erythroid spectrin induces local disorder in the tetramer association region: fluorescence and molecular dynamics studies of free and bound alpha spectrin. Protein Sci. 2009;18:1916–1925. doi: 10.1002/pro.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehboob S, Song Y, Witek M, Long F, Santarsiero BD, Johnson ME, Fung LW-M. Crystal structure of the nonerythroid α-spectrin tetramerization site reveals differences between erythroid and nonerythroid spectrin tetramer formation. J. Biol. Chem. 2010;285:14572–14587. doi: 10.1074/jbc.M109.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehboob S, Luo B-H, Patel BM, Fung LW-M. αβ spectrin coiled coil association at the tetramerization site. Biochemistry. 2001;40:12457–12464. doi: 10.1021/bi010984k. [DOI] [PubMed] [Google Scholar]
- 31.Mehboob S, Jacob J, May M, Kotula L, Thiyagarajan P, Johnson ME, Fung LW-M. Structural analysis of the αN-terminal region of erythroid and nonerythroid spectrins by small-angle X-ray scattering. Biochemistry. 2003;42:14702–14710. doi: 10.1021/bi0353833. [DOI] [PubMed] [Google Scholar]
- 32.Begg GE, Morris MB, Ralston GB. Comparison of the salt-dependent self-association of brain and erythroid spectrin. Biochemistry. 1997;36:6977–6985. doi: 10.1021/bi970186n. [DOI] [PubMed] [Google Scholar]
- 33.Sumandea CA, Fung LW-M. Mutational effects at the tetramerization site of nonerythroid alpha spectrin. Mol. Brain Res. 2005;136:81–90. doi: 10.1016/j.molbrainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acid Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roussigne M, Kossida S, Lavigne A-C, Clouaire T, Ecochard V, Glories A, Amalric F, Girard J-P. The THAP domain: a novel protein motif with similarity to the DNA-binding domain of P element transposase. Trends in Biochem. Sci. 2003;28:66–69. doi: 10.1016/S0968-0004(02)00013-0. [DOI] [PubMed] [Google Scholar]
- 36.Macara IG, Baldarelli R, Field CM, Glotzer M, Hayashi Y, Hsu S-C, Kennedy MB, Kinoshita M, Longtine M, Low C, Maltais LJ, McKenzie L, Mitchison TJ, Nishikawa T, Noda M, Petty EM, Peifer M, Pringle JR, Robinson PJ, Roth D, Russel S, Stuhlmann H, Tanaka M, Tanaka R, Trimble W, Ware J, Zeleznik-Le NJ, Zieger B. Mammalian septins nomenclature. Mol. Biol. Cell. 2002;13:4141–4143. doi: 10.1091/mbc.E02-07-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson EA, Petty EM. Conquering the complex world of human septins: implications for health and disease. Clin. Genet. 2010;77:511–524. doi: 10.1111/j.1399-0004.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han GA, Malintan NT, Collins BM, Meunier FA, Sugita S. Munc18-1 as a key regulator of neurosecretion. J. Neurochem. 2010;115:1–10. doi: 10.1111/j.1471-4159.2010.06900.x. [DOI] [PubMed] [Google Scholar]
- 39.David Y, Ziv T, Admon A, Navon A. The E2 ubiquitin-conjugating enzymes direct polyubiquitination to preferred lysines. J. Biol. Chem. 2010;285:8595–8604. doi: 10.1074/jbc.M109.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ardley HC, Moynihan TP, Markham AF, Robinson PA. Promoter analysis of the human ubiquitin-conjugating enzyme gene family UBE2L1-4, including UBE2L3 which encodes UbcH7. Biochim. Biophys. Acta. 2000;1491:57–64. doi: 10.1016/s0167-4781(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 41.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]