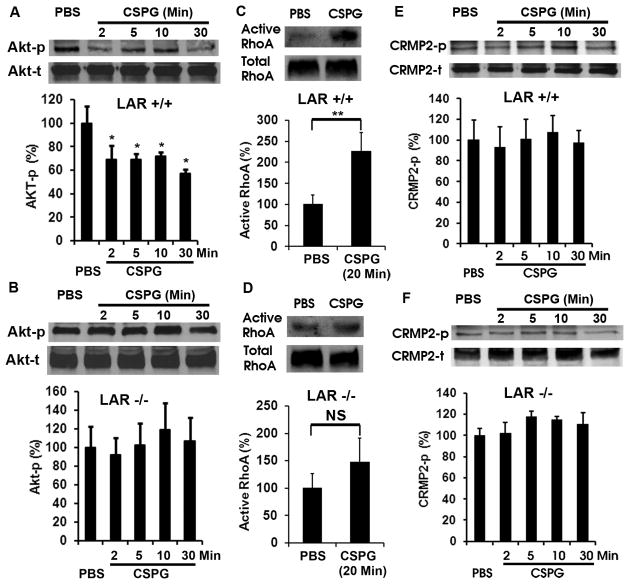
Figure 6. LAR deletion eliminates CSPG-induced Akt inactivation and RhoA activation in CGN cultures.
A, B, Western blots indicate the levels of phosphorylated Akt (Akt-p, active form) in the supernatants of CGNs 24 hrs after cultures. Application of CSPG inhibitor (1.5 μg/ml) significantly reduced the levels of Akt-p 2–30 min after exposures in CGNs derived from LAR +/+ mice (A), although the total protein levels (Akt-t) are similar in the supernatants. However, application of CSPGs did not induce such a change of Akt-p in CGNs derived from LAR −/− mice (B). The differences indicated are compared with PBS control. C, D, RhoA activity (top bands) was determined in cell supernatants of cultured CGNs 24 hrs after growth via RBD precipitation and Western blotting. Total RhoA levels (bottom bands) were determined from the same cell supernatants with blotting. Treatment with CSPGs (1.5 μg/ml) for 20 min significantly increases RhoA activation in neurons (C). Nevertheless, LAR deletion significantly eliminates RhoA activation induced by CSPG stimulation (D). E, F, Western blots indicate the levels of phosphorylated CRMP2 (CRMP2-p, Thr514) in the supernatants of CGNs 24 hrs after cultures. Following 2–30 minute exposures, CSPG (1.5 μg/ml) did not significantly alter the levels of CRMP2-p in CGNs derived from either LAR +/+ or −/− mice. Graphs in A–F at the bottom of the bands indicate the levels of signaling proteins in CGN supernatants quantified from 4 separate experiments. *p < 0.05; **p < 0.01, Student’s t test.