Abstract
Uncontrollable stressors produce behavioral changes that do not occur if the organism can exercise behavioral control over the stressor. Previous studies suggest that the behavioral consequences of uncontrollable stress depend on hypersensitivity of serotonergic neurons in the dorsal raphe nucleus (DRN), but the mechanisms involved have not been determined. We used ex vivo single unit recording in rats to test the hypothesis that the effects of uncontrollable stress are produced by desensitization of DRN 5-HT1A autoreceptors. These studies revealed that uncontrollable, but not controllable, tailshock impaired 5-HT1A receptor-mediated inhibition of DRN neuronal firing. Moreover, this effect was observed only at timepoints when the behavioral effects of uncontrollable stress are present. Furthermore, temporary inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol, which eliminates the protective effects of control on behavior, led even controllable stress to now produce functional desensitization of DRN 5-HT1A receptors. Additionally, behavioral immunization, an experience with controllable stress before uncontrollable stress that prevents the behavioral outcomes of uncontrollable stress, also blocked functional desensitization of DRN 5-HT1A receptors by uncontrollable stress. Lastly, western blot analysis revealed that uncontrollable stress leads to desensitization rather than downregulation of DRN 5-HT1A receptors. Thus, treatments that prevent controllable stress from being protective led to desensitization of 5-HT1A receptors, while treatments that block the behavioral effects of uncontrollable stress also blocked 5-HT1A receptor desensitization. These data suggest that uncontrollable stressors produce a desensitization of DRN 5-HT1A autoreceptors, and that this desensitization is responsible for the behavioral consequences of uncontrollable stress.
Keywords: stress, serotonin, medial prefrontal cortex, dorsal raphe nucleus, single unit recording, learned helplessness
Introduction
The degree of behavioral control that an organism has over a stressor critically determines the behavioral and neurochemical consequences of that stressor. Behavioral control is typically studied in a paradigm in which one subject can behaviorally terminate tailshocks (escapable shock, ES), while another yoked subject cannot (inescapable shock, IS). Many behaviors occur following IS (impaired escape learning, exaggerated anxiety, etc.), that do not occur after ES, despite identical shock delivery (Maier and Watkins, 2005). This phenomenon is termed “learned helplessness” (Maier and Seligman, 1976).
Considerable effort has been devoted to understand why IS and ES produce different behavioral outcomes. Alterations in dorsal raphe nucleus (DRN) serotonergic (5-HT) functioning are clearly involved. IS, relative to ES, intensely activates DRN 5-HT neurons (Grahn et al., 1999), leading to large accumulations of extracellular 5-HT within the DRN (Amat et al., 2005) and projection regions (Bland et al., 2003). This activation sensitizes DRN 5-HT neurons for 24–72 hr. Now, after IS, stimulation of 5-HT neurons, as occurs during behavioral testing, releases exaggerated amounts of 5-HT in projection regions (Amat et al., 1998). Finally, exaggerated 5-HT in projection regions appears responsible for IS behaviors since (i) inhibition of DRN activation during either behavioral testing or IS blocks and prevents, respectively, the expression of IS-induced behaviors (Maier et al., 1995a); (ii) blocking 5-HT receptors in projection regions prevents IS-induced behaviors (Christianson et al., 2010); and (iii) pharmacological activation of DRN 5-HT neurons in the absence of IS produces IS-induced behaviors (Maier et al., 1995b).
Clearly, sensitization of DRN 5-HT neurons is essential in this process. However, the mechanism(s) by which IS sensitizes these neurons is unknown. One possibility is that IS desensitizes DRN inhibitory autoreceptors. The 5-HT1A receptor (5-HT1A-R) is a likely candidate. In the DRN, 5-HT1A-Rs are somatodendritically expressed and, upon activation, inhibit 5-HT cell firing (Sprouse and Aghajanian, 1987) and release (Bonvento et al., 1992). Indeed, reduced 5-HT1A-R expression is associated with excessive 5-HT release in DRN projection regions upon stimulation (Richardson-Jones et al., 2010). Furthermore, acute administration of a 5-HT1A-R agonist can desensitize 5-HT1A-Rs (Beer et al., 1990). As noted above, IS produces large and prolonged elevations of extracellular 5-HT within the DRN. Thus, the conditions for 5-HT1A-R desensitization are present.
Here we investigate whether stressor controllability alters DRN 5-HT1A-R function and expression. We do this by measuring both single unit activity of 5-HT cells in midbrain slices and DRN 5-HT1A-R protein expression. We explore causality between DRN 5-HT1A-R desensitization and IS-produced behaviors by using treatments known to (i) eliminate the protective effects of ES and (ii) block the behavioral effects of IS. Since inactivation of the medial prefrontal cortex (mPFC) during ES eliminates its protective effects on behavior (Amat et al., 2005), then mPFC inactivation during ES should lead ES to desensitize 5-HT1A-Rs. Conversely, since prior exposure to ES blocks the behavioral effects of IS (Amat et al., 2006), then prior ES should also prevent IS-induced 5-HT1A-R desensitization. The experiments below investigate these hypotheses.
Materials & Methods
Subjects
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) aged approximately 2.5-3.5 months and weighing 275–325 g were used in all experiments. Rats were singly housed and maintained on a 12 hr light/dark cycle (lights on at 07:00 and off at 19:00) with food and water provided ad libitum. Rats were allowed at least 7 days to acclimate in the colony room before any surgery or experimentation. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado-Boulder.
Surgery and Cannulation
Rats were anesthetized with isoflurane (Webster Veterinary, Sterling, MA, USA) and a 26-gauge dual guide cannula (Plastics One, Roanoke, VA, USA), 1 mm center-to-center distance, was implanted. The tips of the cannulae were directed 1 mm above the border of the infralimbic (IL) and prelimbic (PL) subregions of the mPFC (AP +2.7 mm, DV −3.3 mm from dura mater, ML 0.5 mm relative to midline) (Paxinos and Watson, 1998). Rats were allowed to recover at least 7 day before experimentation.
Microinjection
Microinjections were delivered with a Kopf Instruments Model 5000 microinjector (Kopf Instruments, Tujunga, CA, USA). A dual 33-gauge microinjector tip (Plastics One) was connected to two 10μl microsyringes with polyethylene 50 tubing (Becton, Dickinson and Company, Sparks, MD, USA). Rats were gently wrapped in a towel and the microinjector tip was inserted into the cannula guides, extending 1 mm beyond the cannula tip. Microinjections were given over the course of 1 min and remained in the cannula for 2 min to ensure drug diffusion. Rats received bilateral microinjections containing 50 ng of muscimol (Sigma, St. Louis, MO, USA) or 0.9% saline at a volume of 0.5 μl per hemisphere. Microinjections were considered successful if following removal from the guide cannula fluid was readily dispensable from injector tips.
Stressor Controllability
Rats were placed in clear Plexiglas boxes (11 x 14 x 17 cm) containing a wheel at the front and a Plexiglas rod extending from the rear. The rat’s tails were taped to the Plexiglas rods and two copper electrodes were affixed to the tail, augmented with electrode paste. Electric shocks were delivered to the rats with a Precision-Regulated Animal Shocker and Graphic State 3.0 software (Coulbourn Instruments, Allentown, PA, USA). The shock protocol consisted of 80 shocks separated by an average inter-trial interval of 60 s. Shock intensity increased every 30 min (1.0 mA, 1.3 mA, 1.6 mA) to maintain reliable escape behavior. Shocks were given in yoked rat pairs (ES and IS); therefore, the shock terminated for both ES and IS rats when the ES rat performed the required operant escape response. The required response at the beginning of the stress session was a ¼ turn of the wheel. The response requirement increased to a ½ wheel-turn following three consecutive trials of ¼ wheel-turns that were performed within 5 sec. Subsequently, the response requirement increased by 50% provided the previous response requirement was performed within 5 sec. The maximum response requirement was 4 full wheel-turns. Notably, the only difference between shock groups was that ES rats controlled the termination of shock. Rats that received no stress were left in their homecages as homecage controls (HC). Rats used in the timecourse and the 5- HT1A-R protein expression experiments received 100 tailshocks at an intensity of 1.0 mA in a Plexiglas restraint tube (17.5 cm in length and 7 cm in diameter) with an average inter-trial interval of 60 s. Restraint tubes, rather than wheel-turn boxes, were used for tailshock in the timecourse and protein expression experiments because stressor controllability was not manipulated.
Rats in the “behavioral immunization” experiment were assigned to 1 of 3 treatment groups. The behavioral immunization group (ES/IS) received ES on Day 1 as described above, and 24 hr later on Day 2 received IS. IS on Day 2 consisted of 100 tailshocks in a Plexiglas restraint tube (17.5 cm in length and 7 cm in diameter) at an intensity of 1.0 mA with an average inter-trial interval of 60 s. The IS treatment group (HC/IS) received HC treatment on Day 1 and IS in a restraint tube on Day 2. Lastly, an unstressed group (HC/HC) remained in their homecages on Days 1 and 2. A treatment group that received IS on both Day 1 and Day 2 (IS/IS group) was not included as previous comparisons to the HC/IS group revealed no differences (Amat et al., 2006). In all experiments, midbrain slices were taken 24 hr following the last shock treatment.
Brain Slice Preparation
Under sodium pentobarbital (60 mg/kg, i.p.), rats were decapitated and the brain was rapidly removed, blocked coronally, rostral to the DRN, and affixed to a vibratome stage (DSK Microslicer; DTK-1000; Dosaka EM, Kyoto, Japan) with cyanoacrylate glue. Brains were immersed in cold (4 °C ) artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 3.25 KCl, 2.4 MgSO4, 2 CaCl2, 1.25 KH2PO4, 10 D-glucose, and 26 NaHCO3 bubbled with 95% O2/5% CO2 during slicing. The time from decapitation to immersion in aCSF was never more than 150 s. Midbrain slices (450 μm) containing the DRN were collected starting caudally at about −8.76 mm from Bregma (Paxinos and Watson, 1998). Three consecutive sections of the DRN were taken, with the most rostral slice ending approximately at −7.80 mm from Bregma (Paxinos and Watson, 1998). Midbrain slices were placed in a Petri dish containing aCSF (RT) bubbled with 95% O2/5% CO2 and allowed to equilibrate for at least 1 hr. Following equilibration, midbrain slices were placed on a sloping liquid-gas interface perfusion chamber for recording. The slope was lined with a double-layer of lens tissue paper underneath the slice and tissue paper flanking the four sides of the slice to maintain hydration. Slices in the chamber were heated at 35 ± 1 °C and perfused with oxygenated aCSF containing 3 μM of phenylephrine hydrochloride (an α1 adrenergic receptor agonist) at a flow rate of 750 μl per min. Phenylephrine hydrochloride was added to increase 5-HT neuronal firing rates to levels observed in vivo (VanderMaelen and Aghajanian, 1983) because noradrenergic afferents to the DRN are severed during slicing. Since the strategy of the experiments below was to assess feedback inhibition of baseline 5-HT cell firing rates, restoration of spontaneous 5-HT firing rates with an α1 adrenergic agonist was essential. Additionally, despite the fact that axonal projections of 5-HT neurons are severed in this preparation, neurons in the DRN remain viable and maintain firing properties that are characteristic of in vivo raphe cell activity (Mosko and Jacobs, 1976).
Extracellular Recording
Extracellular recordings were made with borosilicate glass pipettes filled with aCSF. The glass electrode was connected to an alternating current differential preamplifier (x1000) and visualized in a window discriminator. Units were screened for characteristics consistent with a serotonergic phenotype (VanderMaelen and Aghajanian, 1983). All cells were preferentially sampled from the mid-rostrocaudal to caudal (~ −7.80 mm to −8.30 mm, relative to Bregma) dorsomedial DRN, as this region has several efferents to anxiety- and fear-related structures (Lowry et al., 2008). Once isolated, activity of a unit was recorded with Spike2 software (version 5.05, Cambridge Electronics Design, Cambridge, UK) for 5 min to assess the baseline firing rate. Slices were then perfused with 50μM of 5-HT for 2 min. Units that were reversibly inhibited by 5-HT and expressed characteristics consistent with the 5-HT cell phenotype, such as long duration biphasic or triphasic action potentials, a regular firing pattern, and a firing rate approximately ranging from 0.5 Hz to 2.5 Hz (VanderMaelen and Aghajanian, 1983; Burlhis and Aghajanian, 1987), were deemed putative 5-HT cells. Following recovery from application of 50 μM 5-HT, a variety of drugs were applied to the slice and changes in firing rate were calculated.
Drugs
For mPFC microinjections, muscimol (Sigma) was dissolved in 0.9% saline according to required dose. The 5-HT1A-R antagonist WAY 100635 (N-[2-[4-(2- methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexanecarboxamide trihydrochloride) and 5-hydroxytryptamine hydrochloride were purchased from Sigma and aliquoted with aCSF. The 5-HT1A-R agonist ipsapirone (2-[4-[4-(2-pyrimidinyl)-1- piperazinyl]butyl]-1,2-benzis othiazol-3(2H)-one-1,1-dioxide) was purchased from Tocris Bioscience (Ellisville, MO, USA) and aliquoted in dimethyl sulfoxide (Calbiochem, San Diego, CA, USA). Importantly, ipsapirone is a selective 5-HT1A-R agonist in the DRN while a partial agonist in other brain regions (Glaser et al., 1985; Dong et al., 1997). Unless otherwise noted, all drugs used for ex vivo extracellular recordings were applied for 2 min and dissolved in aCSF containing phenylephrine hydrochloride (Sigma) during slice application.
Analysis of Firing Rates and Responses
Unless otherwise noted, the dependent measure used throughout all experiments was mean ‘percent inhibition’. Percent inhibition was calculated by determining the mean firing rate during the 2 min prior to drug application, ‘mean baseline’. The mean firing rate during drug perfusion was calculated from the time of drug application until maximal inhibition of firing, the ‘mean drug firing rate’. Therefore, the percent inhibition was calculated as (1–(mean drug firing rate/mean baseline)) x 100. Mean percent inhibition was chosen as the dependent measure because it accounts for the rate at which a cell becomes maximally inhibited. Other dependent measures such as 'mean maximal inhibition' do not account for the rate at which a cell reaches maximal inhibition. All drugs were applied for 2 min unless otherwise noted. Stress-induced alterations of baseline firing rates were analyzed by comparing the mean baseline firing rate 2 min prior to application of 50 μM 5-HT, as 50 μM 5-HT was applied to all cells as a test of a serotonergic phenotype.
Histology
To verify cannula location, brains were frozen by placement over dry ice and then stored at 80 °C. Coronal slices measuring 40 μm in thickness were taken throughout the frontal cortex and mounted on gelatin-coated glass slides. Sections were stained with cresyl violet and examined for cannula tracts under light microscopy. Rats with cannula tips outside of IL or PL subregions were excluded from analysis.
Western blot
Rats were sacrificed 24 hr following IS or HC treatments. An ES group was not included because 5-HT1A-R-mediated inhibitions were similar between HC and ES groups. Following an injection of sodium pentobarbital (60 mg/kg, i.p.), rats were decapitated and brains were rapidly dissected, placed in ice-cold isopentane, and stored at −80 °C. Frozen brains were mounted in a cryostat and micropunches measuring ~1 mm3 were taken from the DRN, centered on the dorsomedial DRN (AP −7.5 to −8.5 mm, DV −5.4 mm from dura mater, ML 0.0 mm) and sonicated in a cocktail containing extraction buffer (Invitrogen, Carlsbad, CA, USA) and protease inhibitors (Sigma). Ice-cold tissue samples were centrifuged at 10,000 rpm for 5 min. The resulting supernatant was removed and the protein concentration of each sample was quantified using the Bradford method.
Samples were heated to 70 °C for 10 min and protein samples were loaded into a standard polyacrylamide gel (Bis-Tris Gel, Invitrogen). Electrophoresis was performed in MOPS running buffer at 150 V for 1.5 hr using the XCell SureLock Mini-Cell (Invitrogen). Protein was transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) for 75 min at 40 V. The membrane was blocked with Odyssey blocking buffer (Li-COR Biosciences, Lincoln, NE) for 1.5 hr and incubated with 5-HT1A-R antibody (1:500) (ab44635, Abcam, Cambridge, MA, USA) in blocking buffer overnight at 4 °C. The following day, the membrane was washed in PBS containing Tween (0.1%) and then incubated in blocking buffer containing goat anti-rabbit IRDye 800CW secondary antibody (1:10,000) (926-32211; Li-COR Biosciences) for 45 min at RT. Protein expression was visualized using an Odyssey Infrared Imager (Li-COR Biosciences). Following imaging of 5-HT1A-R expression, the membrane was stripped and re-probed to assess whether IS altered the expression of the sodium-potassium ATPase (Na-K ATPase) (1:5,000) (ab7671, Abcam), a housekeeping protein. The following day, the membrane was washed in PBS containing Tween (0.1%) and then incubated in blocking buffer containing goat anti-mouse IRDye 800CW secondary antibody (1:10,000) (926-32210; Li-COR Biosciences) for 45 min at RT. Protein expression was quantified using Odyssey imaging software (Li-COR Biosciences) and data were expressed as a ratio of 5- HT1A-R / Na-K ATPase expression, normalized to HC.
Statistics
All data are expressed as mean ± SEM. In experiments assessing dose-response, stress was a between-subjects factor and drug dose was a within-subjects factor. Percent inhibitions of firing rate were analyzed as independent observations using an unpaired t-test, one-way ANOVA, or two-way ANOVA, as appropriate. Statistically significant main effects were followed by either Dunnett’s Multiple Comparison test or Fisher’s Protected Least Significant Difference post hoc analysis (two-tailed α = 0.05).
Results
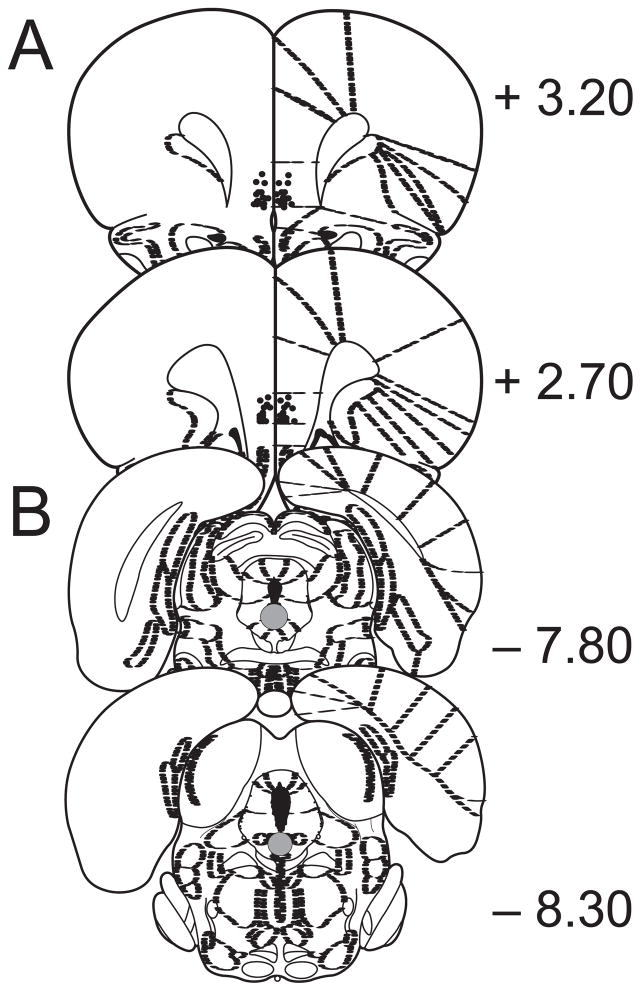
Figure 1 shows cannula placements within the mPFC for Experiment 5 and the region of the DRN where 5-HT cell recordings were preferentially taken throughout all experiments. All cannulated subjects had microinjector tips terminating in either the IL or PL regions of the mPFC.
Figure 1.
A, Placements of microinjection cannulae in the medial prefrontal cortex. Black circles indicate the location of the cannulae tips. B, Location of single unit extracellular recordings in the dorsal raphe nucleus. The gray-shaded areas indicate where recordings were preferentially taken. Numerals indicate distance from Bregma in millimeters. Anatomical maps adopted from Paxinos and Watson (1998).
Experiment 1: Effect of stressor controllability on serotonin-mediated inhibition of DRN 5-HT cell firing
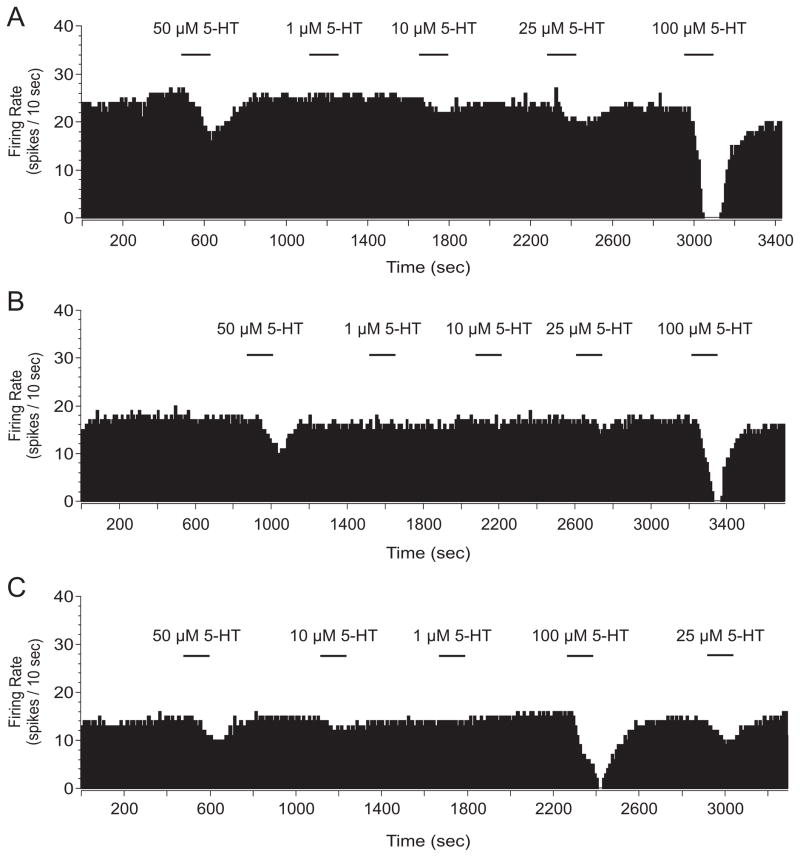
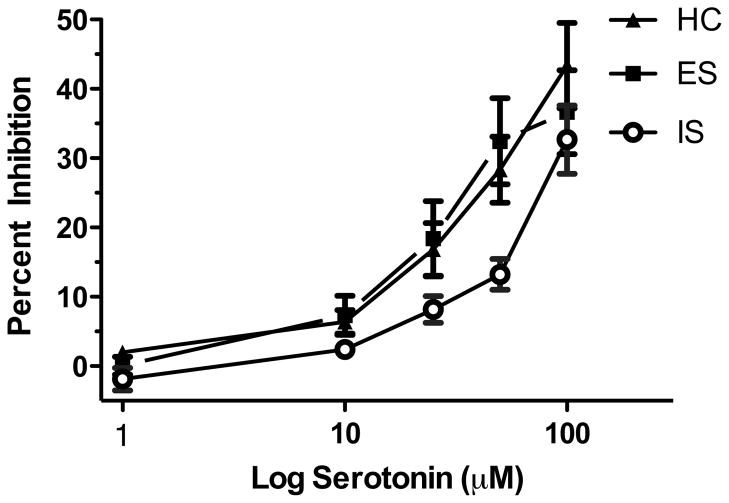
Rats received either ES, IS, or HC treatment and were sacrificed 24 hr later for extracellular single unit recording. To determine if IS desensitized 5-HT1A-Rs in the DRN the endogenous ligand for the 5-HT1A-R, 5-HT, was used. Varying doses of 5-HT (1, 10, 25, 50, and 100 μM) were applied for 2 min and percent inhibition was calculated. As shown in Figures 2 and 3, serotonin-mediated inhibition of DRN 5-HT cell firing was impaired following IS, but not following ES. This result was confirmed by a two-way ANOVA that revealed significant main effects of stress (F(2, 248) = 6.410; p < 0.01) and dose (F(4, 248) = 38.530; p < 0.0001), but no significant interaction. Subsequent post hoc comparisons revealed that IS rats were significantly different from ES and HC rats. Importantly, the ES and HC post hoc comparison was not significant. Stress-induced changes of baseline firing rate were analyzed but no significant differences were found (F(2, 58) = 3.008; p > 0.05). Indeed, we did not expect to observe stress-induced differences in firing rate because tonic 5-HT1A-R-mediated autoinhibition of 5-HT cell firing rates in an in vitro preparation is dependent on exogenous tryptophan availability (Evans et al., 2008).
Figure 2.
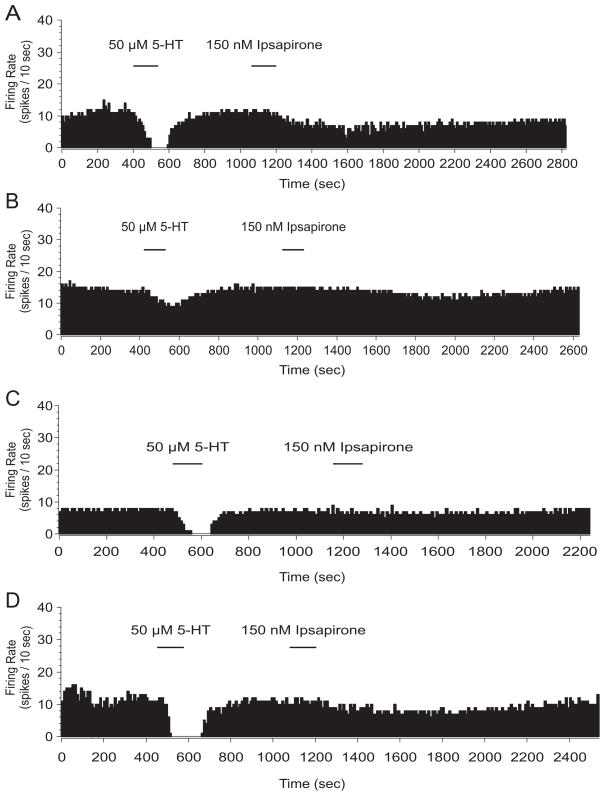
Spike frequency histograms from extracellular single unit recording in the dorsal raphe nucleus following homecage control (A), escapable stress (B), and inescapable stress (C). Midbrain slices were taken 24 hr after stress treatment and varying doses of serotonin (5-HT) were applied. Mean percent inhibition was calculated to account for the rate at which the cell reaches maximal serotonin-mediated inhibition.
Figure 3.
Inescapable stress (IS) selectively impairs serotonin-mediated inhibition of dorsal raphe nucleus (DRN) cell firing 24 hr following stress treatment. The graph depicts the effect of stress treatment on serotonin-mediated inhibition of DRN cell firing. Groups are designated as the following: homecage control (HC), closed triangles; escapable stress (ES), closed squares; IS open circles. Data are expressed as mean percent inhibition ± SEM. Mean percent inhibition of DRN cell firing was significantly different in IS, compared to ES and HC (p < 0.05).
Experiment 2: Role of the 5-HT1A-R during serotonin-mediated inhibition of DRN 5-HT cell firing
Although 5-HT is the endogenous ligand for 5-HT receptors, the DRN expresses several 5-HT receptor classes (Barnes and Sharp, 1999). To determine the role of the 5-HT1A-R during serotonin-mediated inhibition of DRN cell firing, the 5-HT1A-R antagonist WAY 100635 was used. Slices of the midbrain were taken from experimentally naïve rats for extracellular single unit recording. After 5 min of baseline firing rate collection, 100 μM of 5-HT was applied for 2 min and percent inhibition was calculated. Following recovery from 100 μM 5-HT, 20 nM of WAY 100635 was applied to the slice for 16 min. After superfusion with WAY 100635, a cocktail of 20 nM of WAY 100635 and 100 μM of 5-HT was applied to the slice for 2 min and percent inhibition was calculated. This protocol has been used by others to assess the contribution of the 5-HT1A-R during serotonin- mediated inhibition in the rat DRN (Fairchild et al., 2003), although in this previous study, recordings were not targeted specifically to the dorsomedial DRN. WAY 100635 blocked serotonin-mediated inhibition of cell firing in the dorsomedial DRN (data not shown). An unpaired t-test revealed a significant difference between mean percent inhibition of the 5-HT applications (t(12) = 3.230; p < 0.01).
Experiment 3: Effect of inescapable stress on ipsapirone-mediated inhibition of DRN 5- HT cell firing
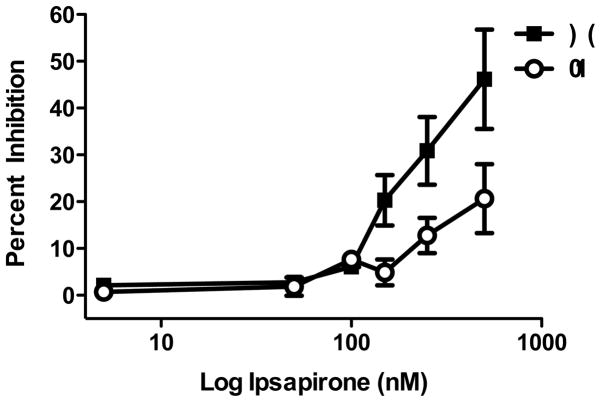
Rats received either IS or HC treatment and were sacrificed 24 hr later for extracellular single unit recording. Experiment 3 did not contain an ES group as Experiment 1 revealed no significant differences between ES and HC rats. The 5-HT1A-R agonist ipsapirone was applied at varying doses (5, 50, 100, 150, 250, and 500 nM) for 2 min and percent inhibition was calculated. As shown in Figure 4, ipsapirone-mediated inhibition of DRN cell firing was impaired in IS rats at a number of doses. These results were confirmed by a two-way ANOVA. The effect of stress (F(1, 161) = 16.32; p < 0.001), dose (F(5, 161) = 13.76; p < 0.001) and interaction of stress x dose (F(5, 161) = 3.27; p < 0.01) were significant. Subsequent post hoc comparisons indicated that IS was significantly different from HC. Additionally, post hoc analysis of stress x dose indicated that IS and HC were significantly different at the 150 and 250 nM ipsapirone doses. Again, stress had no significant effect on baseline firing rate (t(68) = 0.8031; p > 0.05).
Figure 4.
Inescapable stress (IS) impairs ipsapirone-mediated inhibition of dorsal raphe nucleus cell firing 24 hr following stress treatment. Groups are expressed as the following: closed squares, homecage control (HC); open circles, IS. Data are expressed as mean percent inhibition ± SEM. IS significantly reduced mean percent inhibition of ipsapirone-mediated inhibition of DRN cell firing as compared to HC (p < 0.001). *Mean ipsapirone-mediated inhibition was significantly different between IS and HC at the 150 and 250 nM doses (p < 0.05).
Experiment 4: Timecourse of IS effects on ipsapirone-mediated inhibition of DRN 5-HT cell firing
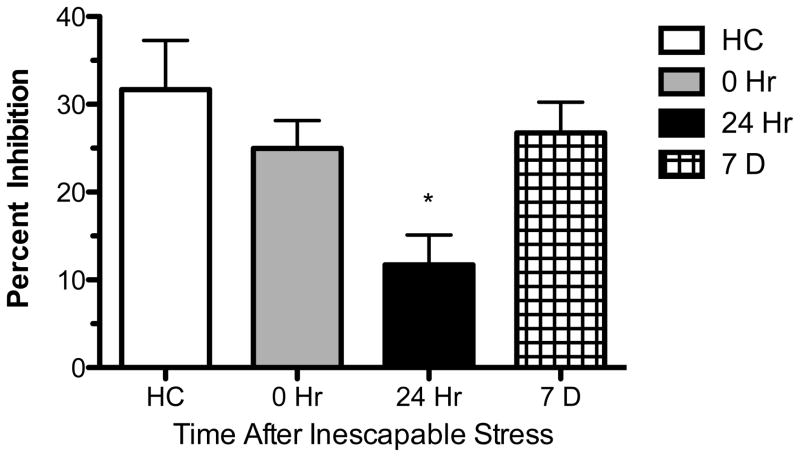
Rats received IS in a Plexiglas restraint tube or remained in the colony as HC controls. Rats were sacrificed either immediately following IS (0 hr), 1 day later (24 hr), or 1 week later (7 D) for extracellular single unit recording. These time-points were chosen because the behavioral effects of IS are observed between 24–72 hr following IS exposure (Glazer and Weiss, 1976; Grau et al., 1981; Maier, 1990). Additionally, since Experiment 3 revealed a significant difference between IS and HC rats at the 150 nM dose of ipsapirone, only the 150 nM dose was used as it was the lowest dose that produced a maximal difference between IS and HC rats. As shown in Figure 5, ipsapirone-mediated inhibitions were significantly impaired 24 hr following IS. A one-way ANOVA revealed (F(3, 48) = 3.977; p < 0.05) a significant stress group effect and a subsequent Dunnett’s Multiple Comparison test revealed a significant difference between the IS 24 hr time-point and HC. The 0 hr and 7 D time-points were not significantly different from HC. No stress-induced changes in baseline firing rate were observed (F(3, 48) = 0.8020; p > 0.05).
Figure 5.
Ipsapirone-mediated inhibition of dorsal raphe nucleus serotonin cell firing is selectively impaired 24 hr following inescapable stress (IS). The white bar indicates non-stressed homecage control (HC), the gray bar indicates IS rats that were sacrificed immediately following IS, the black bar indicates rats sacrificed 24 hr after IS, and the patterned bar represents rats that were sacrificed 7 days (D) after IS. The data are expressed as mean percent inhibition ± SEM. Mean percent inhibition was significantly different among groups (p < 0.05). *Dunnett's Multiple Comparison test revealed that only the 24 hr time-point was significantly different from HC (p < 0.05).
Experiment 5: Effect of mPFC inactivation during stress on ipsapirone-mediated inhibition of DRN 5-HT cell firing
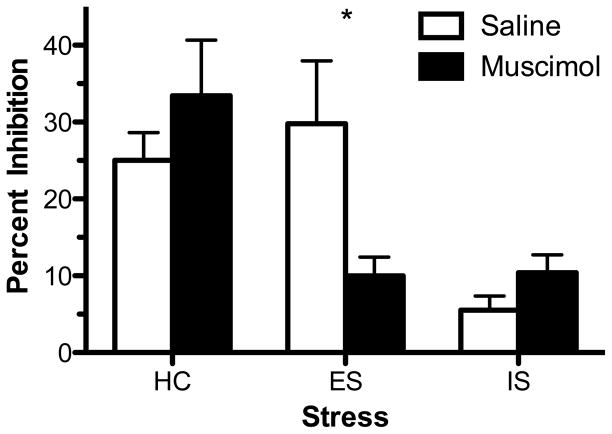
Rats received intra-mPFC saline or 50 ng muscimol 45 min before ES, IS, or HC treatment, 24 hr later midbrain slices were taken for single unit recording. Importantly, intra-mPFC muscimol does not interfere with escape learning during ES (Amat et al., 2005). As shown in Figures 6 and 7, impairment of 5-HT1A-R-mediated inhibition was found in IS rats as compared to HC at 150 nM of ipsapirone. Intra-mPFC muscimol had no effect on IS subjects. However, intra-mPFC muscimol led ES to now impair ipsapirone-mediated inhibition of firing. A two-way ANOVA revealed a significant effect of stress (F(2, 57) = 8.08; p < 0.001) and stress x muscimol (F(2, 57) = 4.57; p < 0.05) interaction. The effect of muscimol (F(1, 57) = 0.26; p > 0.05) was not significant. Subsequent post hoc comparisons revealed a significant difference between muscimol-and saline-ES rats. Importantly, post hoc comparisons also revealed (i) no difference between muscimol-ES and muscimol-IS groups (ii) no difference between saline-ES and saline-HC groups (iii) a significant difference between muscimol-ES and muscimol-HC groups (iv) and a significant difference between IS and HC groups treated with either saline or muscimol. All comparisons between stress groups revealed no significant differences in baseline firing rate.
Figure 6.
Spike frequency histograms from extracellular single unit recording in the dorsal raphe nucleus following intra-medial prefrontal cortex (mPFC) injection of muscimol during homecage control (HC) (A), intra-mPFC muscimol during inescapable stress (IS) (B), intra-mPFC muscimol during escapable stress (ES) (C), and intra-mPFC saline during ES (D). Midbrain slices were taken 24 hr after stress treatment and 50 μM of serotonin (5-HT) was applied followed by 150 nM of ipsapirone. Mean percent inhibition was calculated to account for the rate at which the cell reaches maximal ipsapirone-mediated inhibition.
Figure 7.
A microinjection of muscimol into the medial prefrontal cortex (mPFC) during escapable stress (ES) impairs inhibition of dorsal raphe nucleus (DRN) serotonin (5-HT) cell firing with 150 nM ipsapirone. Open bars indicate rats that received intra-mPFC saline, closed bars indicate rats that received intra-mPFC muscimol microinjections. Data are expressed as mean percent inhibition ± SEM. Mean percent inhibition of DRN 5-HT cell firing was significantly different between inescapable stress (IS) and homecage (HC) treatments (p < 0.05). *Mean percent inhibition of DRN 5-HT cell firing was significantly different between ES rats receiving intra-mPFC muscimol and saline (p < 0.05).
Experiment 6: The effect of behavioral immunization on ipsapirone-mediated inhibition of DRN 5-HT cell firing
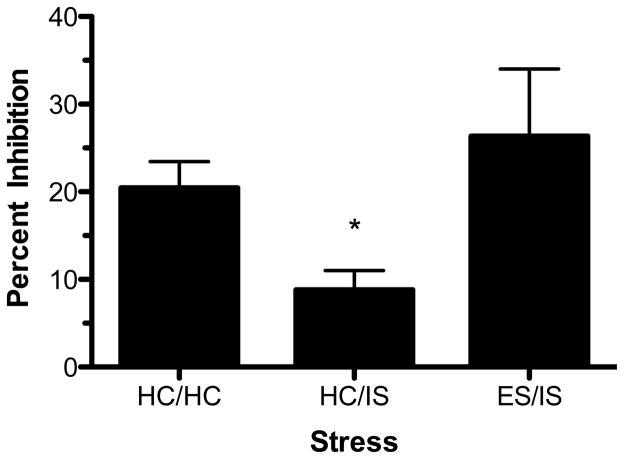
Since prior studies have shown that an experience with ES before IS (so-called “behavioral immunization”) blocks the behavioral and neurochemical consequences of IS (Williams and Maier, 1977; Amat et al., 2006), we tested whether ES prior to IS would block IS-induced functional desensitization of DRN 5-HT1A-Rs. Rats received behavioral immunization or experimental control treatments (see Materials and Methods for detail) and midbrain slices were taken 24 hr later. Again, only the 150 nM dose of ipsapirone was used. As shown in Figures 8 and 9, IS again reduced ipsapirone-mediated inhibition of cell firing, but this reduction was blocked by prior ES. This conclusion was confirmed by a one-way ANOVA (F(2, 41) = 4.497; p < 0.05). Subsequent post hoc comparisons revealed that the HC/IS group was significantly different from the ES/IS and HC/HC groups; however, no significant difference was found between the ES/IS and HC/HC groups. Lastly, stress-induced changes in baseline firing rate were analyzed and no significant differences were found (F(2, 41) = 0.06547; p > 0.05).
Figure 8.
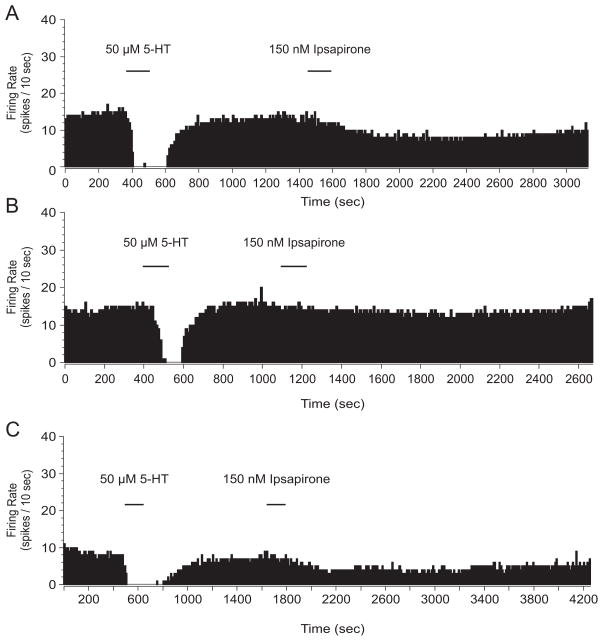
Spike frequency histograms from extracellular single unit recording in the dorsal raphe nucleus (DRN) 24 hr following homecage control (HC/HC) (A), homecage control followed 24 hr later by inescapable stress (HC/IS) (B), and escapable stress followed 24 hr later by inescapable stress (ES/IS) (C). Midbrain slices were taken 24 hr after final stress treatment and 50 μM of serotonin (5-HT) was applied followed by 150 nM of ipsapirone. Mean percent inhibition was calculated to account for the rate at which the cell reaches maximal ipsapirone-mediated inhibition.
Figure 9.
Behavioral immunization blocks inescapable stress (IS)-induced impairment of dorsal raphe nucleus (DRN) serotonin (5-HT) cell firing with 150 nM ipsapirone. Data are expressed as mean percent inhibition ± SEM. Mean percent inhibition was different among stress groups (p < 0.05). *Mean percent inhibition of DRN 5-HT cell firing was significantly different in rats that received homecage treatment followed by IS (HC/IS) as compared to rats that received no stress (HC/HC) and behavioral immunization (ES/IS) (p < 0.05).
Experiment 7: The effect of IS on the expression of 5-HT1A-R protein in the DRN
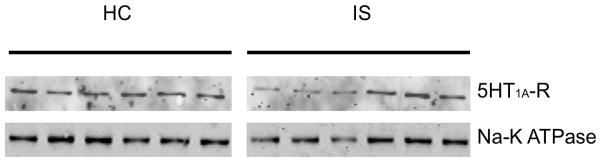
The mechanism by which IS impairs 5-HT1A-R-mediated inhibitions was explored by assessing whether IS selectively downregulates 5-HT1A-R protein expression in the DRN 24 hr following IS and HC. The 24 hr time-point was chosen as this is when the electrophysiological differences between HC and IS are observed. Figure 10 is a representative western blot showing protein expression of the 5-HT1A-R and Na-K ATPase. There was no significant difference in 5-HT1A-R protein expression following IS and HC treatments (t(30) = 0.4207; p > 0.05).
Figure 10.
Protein expression of the serotonin-1A receptor (5-HT1A-R) in the dorsal raphe nucleus is not changed 24 hr following inescapable stress (IS) and homecage (HC) treatments. Representative western blot of 5-HT1A-R and sodium-potassium ATPase (Na- K ATPase) expression in the dorsal raphe nucleus 24 hr following inescapable stress (IS) and homecage (HC) treatments. No significant difference was observed between HC and IS treatments (p > 0.05).
Discussion
Behavioral and neurochemical outcomes following ES and IS are often quite different. In several fear- and anxiety-related tests ES subjects resemble HC controls, while IS subjects exhibit a fear/anxiety phenotype (Maier and Watkins, 2005). Similarly, IS induces several neurochemical changes that do not occur following ES (Maswood et al., 1998). Several studies have shown that IS, relative to ES, activates 5-HT neurons in the DRN (Grahn et al., 1999; Amat et al., 2005), resulting in the sensitization of these neurons to subsequent input (Amat et al., 1998; Bland et al., 2003; Christianson et al., 2010). Moreover, a number of studies have shown that this process is necessary and sufficient to produce typical IS behaviors (Maier et al., 1995b; Maier et al., 1995a; Will et al., 2004; Christianson et al., 2008). However, the mechanism(s) responsible for IS-induced DRN 5-HT sensitization is unexplored. The present experiments examined whether IS, relative to ES, reduces 5-HT feedback inhibition on 5-HT cells, an outcome that would sensitize these cells. Sensitization would occur if IS reduced DRN 5-HT1A-R function. However, reduced 5-HT1A-R function would not, by itself, indicate that this consequence of IS causally mediates IS behaviors.
To investigate the possibility that 5-HT1A-R changes are casual, two strategies were adopted. The first was to determine whether a manipulation that eliminates the protective effects of control on behavior, i.e. a manipulation that produces IS-like behavior in an ES rat, would now lead ES to also reduce inhibition of DRN cells, as does IS. The second was to determine whether a manipulation known to block the behavioral effects of IS would also block the effects of IS on 5-HT1A-R inhibition of 5-HT activity. This type of co-variation is necessary for implicating a mediational role. Ex vivo extracellular single unit recording was used as it directly assesses function, and the effects of drug application with multiple doses can be readily examined.
In Experiment 1 we demonstrated that IS, but not ES, impairs serotonin-mediated inhibition of 5-HT cell firing. Although Experiment 1 has limitations because 5-HT is not selective for the 5-HT1A-R, the use of 5-HT rather than a selective 5-HT receptor agonist most closely mimics in vivo 5-HT release. For this reason Experiment 2 was designed to determine whether 5-HT inhibits DRN 5-HT cell firing primarily via the 5-HT1A-R. Here, a selective 5-HT1A-R antagonist blocked the inhibitory effects of 5-HT on DRN cell firing, suggesting that 5-HT inhibition is exerted primarily via the 5-HT1A-R. Furthermore, Experiment 3 utilized the 5-HT1A-R agonist ipsapirone to inhibit cell firing. Again, prior IS interfered with the neuronal inhibition. Lastly, the timecourse in Experiment 4 demonstrated that impaired 5-HT1A-R-mediated inhibition follows the same timecourse as the behavioral effects of IS. Together these experiments indicate that IS, relative to ES, interferes with serotonin-mediated inhibition of DRN neuronal activity 24 hr later, an effect likely mediated by reduced 5-HT1A-R function.
However, the occurrence of IS-induced reductions in 5-HT inhibition of DRN activity does not indicate that this change is responsible for the behavioral effects of IS. If this change is causal, then manipulations that block the protective effects of control on behavior should eliminate the lack of effect of ES on DRN 5-HT1A-R sensitivity. Several studies demonstrate that the mPFC inhibits DRN 5-HT activity during ES but not during IS. Thus, inactivation of the mPFC with muscimol during ES eliminates the protective effects of ES; consequently, ES now produces the same behavioral outcomes as does IS (Amat et al., 2005; Rozeske et al., 2009). If reduced 5-HT1A-R sensitivity is causal, then inhibition of the mPFC during stress should lead both IS and ES to now interfere with DRN unit inhibition. Experiment 5 explored exactly this prediction. Muscimol was microinjected at the IL-PL border as in Amat et al. (2005), and intra-mPFC muscimol during stress led ES to reduce ipsapirone-mediated inhibition to the same degree as IS.
A causal role for reduced 5-HT inhibition of DRN neurons would also predict the converse. Namely, manipulations known to prevent the behavioral effects of IS should also eliminate the IS-induced impairment of 5-HT1A-R inhibition. A number of studies (Williams and Maier, 1977; Amat et al., 2006) have shown that prior experience with ES blocks the behavioral effects of subsequent IS, a phenomenon labeled behavioral immunization. As a causal role for reduced inhibition of DRN neurons would predict, prior ES eliminated IS-induced reduction in ipsapirone-mediated inhibition of unit activity. Using a similar strategy, voluntary exercise was shown to also block the behavioral effects of IS by increasing DRN 5-HT1A-R mRNA (Greenwood et al., 2003). These data together support the idea that reduced 5-HT1A-R function is part of the causal network that mediates the behavioral effects of IS.
To establish causality it would also be desirable to determine whether manipulations which reduce DRN 5-HT1A-R inhibitory function, in the absence of IS treatment, also produce typical IS-induced behaviors. Indeed, acute administration of fluoxetine produces typical IS-induced behaviors, 24 hr after injection (Greenwood et al., 2008). Although not measured by Greenwood et al. (2008), acute fluoxetine is known to cause 5-HT1A-R internalization in the DRN (Riad et al., 2004). Finally, it would be desirable to determine whether preventing 5-HT1A-R desensitization during IS blocks the behavioral effects of IS. Essentially, the behavioral immunization experiment above provides such data. In addition it should be noted that pharmacological blockade of DRN 5-HT activation during IS, which would prevent 5-HT1A-R desensitization since extracellular DRN 5-HT would not rise, blocks the behavioral effects of IS (Maier et al., 1995a).
The present experiments used ex vivo extracellular single unit recording in the DRN to investigate the relationship between stressor controllability and 5-HT receptor sensitivity. Others have also used this approach to assess the consequences of a stress experience on receptor function (Laaris et al., 1999; Froger et al., 2004). Although it is remarkable that the effects of stressor controllability carried into the slice preparation, it should be noted that single unit recording has limitations. Although 33–66% of cells in the DRN are serotonergic, extracellular single unit recording cannot definitively identify cell type. Furthermore, despite the well-documented firing characteristics of 5-HT cells (VanderMaelen and Aghajanian, 1983), these characteristics may be insufficient for serotonergic cell identification (Kirby et al., 2003).
The present experiments also demonstrate that impaired ipsapirone-mediated inhibition 24 hr following IS is not due to degradation of 5-HT1A-Rs in the DRN since 5- HT1A-R protein expression was similar 24 hr following IS and HC. Functional desensitization of 5-HT1A-Rs could occur via multiple mechanisms including G protein uncoupling (Flugge, 1995; Laaris et al., 1999; Hensler, 2002), receptor phosphorylation (Lembo and Albert, 1995), reduced K+ channel function (Kobayashi et al., 2006), and reduced G protein expression (Li et al., 1996). However, the western blot data presented in Experiment 7 cannot dissociate these possibilities.
One mechanisms by which stress can produce functional desensitization of DRN 5-HT1A-Rs is via glucocorticoid receptor stimulation (Laaris et al., 1995). This is an unlikely mechanism for the stress-group differences observed here, as the ES and IS treatments used cause similar release of glucocorticoids (Maier et al., 1986). However, IS and ES do lead to very different levels of extracellular 5-HT within the DRN (Maswood et al., 1998; Amat et al., 2005), with IS producing large and sustained elevations. Since agonism of the 5-HT1A-R has been shown to desensitize 5-HT1A-Rs (Beer et al., 1990; van Huizen et al., 1993; Harrington et al., 1994; Riad et al., 2004), this would be a likely cause. Furthermore, inhibition of the mPFC with muscimol during the stressor not only leads ES to produce the behavioral outcomes normally produced by only IS, but it also leads ES to produce the same high levels of extracellular 5-HT within the DRN as does IS (Amat et al., 2005). Conversely, a prior experience with ES not only blocks the behavioral effects of later IS, but it also prevents the IS-induced increase in extracellular 5-HT within the DRN (Amat et al., 2006). Thus, whether 5-HT1A-R desensitization was or was not produced by a given manipulation in the present studies is perfectly predicted by whether the manipulation does or does not lead to elevated levels of extracellular 5-HT within the DRN.
The functional desensitization of DRN 5-HT1A-Rs following IS resembles clinical findings reporting a relationship between raphe 5-HT1A-R alterations and anxiety- and depression-related psychopathologies. Indeed, patients with depression show reduced binding of the 5-HT1A-R in the raphe (Drevets et al., 1999; Meltzer et al., 2004; Drevets et al., 2007). Additionally, reduced binding of 5-HT1A-Rs in the raphe is also observed in social anxiety disorder (Lanzenberger et al., 2007) and panic disorder (Nash et al., 2008). These clinical findings encourage the notion that IS may capitulate some of the endophenotypes associated with depression and anxiety.
Acknowledgments
We would like to thank J. Christianson and T. Doyle for commentary & technical assistance and the National Institutes of Health for financial support (grants DA023329 (RRR), MH086539 (CAL), and MH050479 (SFM)).
References
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beer M, Kennett GA, Curzon G. A single dose of 8-OH-DPAT reduces raphe binding of [3H]8-OH-DPAT and increases the effect of raphe stimulation on 5-HT metabolism. Eur J Pharmacol. 1990;178:179–187. doi: 10.1016/0014-2999(90)90473-j. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Scatton B, Claustre Y, Rouquier L. Effect of local injection of 8-OH-DPAT into the dorsal or median raphe nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett. 1992;137:101–104. doi: 10.1016/0304-3940(92)90308-t. [DOI] [PubMed] [Google Scholar]
- Burlhis TM, Aghajanian GK. Pacemaker potentials of serotonergic dorsal raphe neurons: contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1:582–588. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, de Montigny C, Blier P. Effect of acute and repeated versus sustained administration of the 5-HT1A receptor agonist ipsapirone: electrophysiological studies in the rat hippocampus and dorsal raphe. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:303–311. doi: 10.1007/pl00005055. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Evans AK, Reinders N, Ashford KA, Christie IN, Wakerley JB, Lowry CA. Evidence for serotonin synthesis-dependent regulation of in vitro neuronal firing rates in the midbrain raphe complex. Eur J Pharmacol. 2008;590:136–149. doi: 10.1016/j.ejphar.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Leitch MM, Ingram CD. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology. 2003;45:925–934. doi: 10.1016/s0028-3908(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Flugge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez-Casteloot I, Enache M, Maccari S, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Rath M, Traber J, Zilles K, Schleicher A. Autoradiographic identification and topographical analyses of high affinity serotonin receptor subtypes as a target for the novel putative anxiolytic TVX Q 7821. Brain Res. 1985;358:129–136. doi: 10.1016/0006-8993(85)90956-4. [DOI] [PubMed] [Google Scholar]
- Glazer HI, Weiss JM. Long-Term and Transitory Interference Effects. J Exp Psychol Anim Behav Process. 1976;2:191–201. [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hyson RL, Maier SF, Madden Jt, Barchas JD. Long-term stress-induced analgesia and activation of the opiate system. Science. 1981;213:1409–1411. doi: 10.1126/science.7268445. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 2008;199:209–222. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington MA, Shaw K, Zhong P, Ciaranello RD. Agonist-induced desensitization and loss of high-affinity binding sites of stably expressed human 5-HT1A receptors. J Pharmacol Exp Ther. 1994;268:1098–1106. [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Washiyama K, Ikeda K. Inhibition of G protein-activated inwardly rectifying K+ channels by the antidepressant paroxetine. J Pharmacol Sci. 2006;102:278–287. doi: 10.1254/jphs.fp0060708. [DOI] [PubMed] [Google Scholar]
- Laaris N, Haj-Dahmane S, Hamon M, Lanfumey L. Glucocorticoid receptor-mediated inhibition by corticosterone of 5-HT1A autoreceptor functioning in the rat dorsal raphe nucleus. Neuropharmacology. 1995;34:1201–1210. doi: 10.1016/0028-3908(95)00095-n. [DOI] [PubMed] [Google Scholar]
- Laaris N, Le Poul E, Laporte AM, Hamon M, Lanfumey L. Differential effects of stress on presynaptic and postsynaptic 5-hydroxytryptamine-1A receptors in the rat brain: an in vitro electrophysiological study. Neuroscience. 1999;91:947–958. doi: 10.1016/s0306-4522(98)00674-5. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Albert PR. Multiple phosphorylation sites are required for pathway-selective uncoupling of the 5-hydroxytryptamine1A receptor by protein kinase C. Mol Pharmacol. 1995;48:1024–1029. [PubMed] [Google Scholar]
- Li Q, Muma NA, van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: theory and evidence. J Exp Psychol Gen. 1976;105:3–46. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995a;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995b;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Mosko SS, Jacobs BL. Recording of dorsal raphe unit activity in vitro. Neurosci Lett. 1976;2:195–200. doi: 10.1016/0304-3940(76)90014-8. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology. 2009;34:834–843. doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- van Huizen F, Bansse MT, Stam NJ. Agonist-induced down-regulation of human 5-HT1A and 5-HT2 receptors in Swiss 3T3 cells. Neuroreport. 1993;4:1327–1330. doi: 10.1097/00001756-199309150-00010. [DOI] [PubMed] [Google Scholar]
- VanderMaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotoninergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, Pepin JL, Watkins LR, Maier SF. Electrolytic lesions and pharmacological inhibition of the dorsal raphe nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2004;171:191–198. doi: 10.1007/s00213-003-1572-1. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. J Exp Psychol Anim Behav Process. 1977;3:240–253. [Google Scholar]