Abstract
Cancer is one of the major causes of mortality and morbidity in our health care system. Molecular imaging is an emerging methodology for the early detection of cancer, and the development of exogenous molecular probes that can be labeled for multi-modality imaging is critical to this process. Today, molecular imaging is at crossroad, and new targeted imaging agents are expected to broadly expand our ability to detect pre-malignant lesions. This integrated imaging strategy will permit clinicians to not only localize lesions within the body, but also to visualize the expression and activity of specific molecules. This information is expected to have a major impact on diagnosis, therapy, drug development and understanding of basic cancer biology. At this time, a number of molecular probes have been developed by conjugating various labels to affinity ligands for targeting in different imaging modalities. This review will describe the current status of exogenous molecular probes for optical, nuclear and MRI imaging platforms. Furthermore, we will also shed light on how these techniques can be used synergistically in multi-modal platforms and how these techniques are being employed in current research.
Keywords: molecular probes, contrast agents, affinity ligands, peptides, antibodies, nanoparticles, radioligands, imaging, cancer, optics, PET/SPECT, MRI
1. Introduction
In vivo molecular imaging of cancer has made significant progress due to recent advances in molecular biology, coupled with the rapid development of innovations in imaging instrumentation and probe chemistry. Several modalities have been utilized for targeted imaging in cancer such as ultrasound (US), computed tomography (CT), nuclear (PET/SPECT), magnetic resonance imaging (MRI), and optics [1–6]. These techniques have a significant role on all aspects of cancer, including diagnosis, staging, risk stratification, planning and guidance of therapy, and chemoprevention. The development of novel contrast agents are critical to the use of different imaging modalities. For example, various radioisotopes such as 125I, 99mTc, 64Cu, 111In, and 18F for PET/SPECT, supermagnetic or paramagnetic metals for MRI, and various visible and NIR dyes for optical imaging have been developed. Most of these compounds, however, are non-targeted agents.
Molecular imaging has raised increased interest for the detection and management of cancer and has been defined as the characterization and measurement of the biological process in living animals at the cellular and molecular levels. To achieve truly targeted imaging of specific molecules which exist in relatively low concentrations in living tissues, the imaging techniques must be highly sensitive. Although US, CT and MRI are often considered as molecular imaging modalities, in practice, nuclear and optical imaging are the most relevant to the above definition and are used most frequently because of the high sensitivity and the specificity for target detection that can be achieved. Table 1 compares some of the advantages and disadvantages of the different imaging modalities for in-vivo evaluation of molecular process. Each modality has its own strengths and weaknesses, varying in sensitivity, spatial resolution, temporal resolution, cost, and depth of tissue penetration.
Table 1.
Various modalities used in molecular imaging.
| Modality | Advantages | Disadvantages |
|---|---|---|
| Optical imaging |
|
|
| Nuclear |
|
|
| Magnetic resonance imaging (MRI) |
|
|
Recently, researchers are developing multi-modal strategies that combine optical, MR and nuclear processes for enhanced validation. These integrated techniques aim to validate observed biological phenomena using independent views and to better delineate the localization and expression of molecular markers. Moreover, the combined imaging methods and probes work synergistically to improve sensitivity for the investigation of biological process. The purpose of this review, we describe the most common molecular imaging modalities: nuclear imaging, optical, and magnetic resonance imaging (MRI), and multi-modal imaging and demonstrate the current status of molecular probes.
Each imaging modality mentioned in Table 1 use different exogenously administered contrast agents to generate images with high molecular specificity. Among the exogenous probes, small molecules, fluorescent dyes conjugated with affinity ligands such as peptides, antibodies, aptamers, radionuclide conjugated peptides, nanoparticles and quantum dots have been extensively used in cancer imaging research. Table 2 provides an overview of the general classes of exogenous targeting agents which are frequently employed across all imaging modalities. Progress in this field is becoming more advanced due to multi-disciplinary collaborations among chemists, molecular biologists, clinicians, physicists, and imaging scientists. Today, the molecular imaging community is rapidly developing the performance of imaging modalities and the diversity of molecular probes.
Table 2.
Various ligands for imaging in different molecular imaging modalities.
| Detection ligand | Advantages | Disadvantages |
|---|---|---|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
2. Scope of the Review
Recent research has demonstrated the development and application of various imaging agents in different imaging modalities. This includes bioluminescent reporters, protease-sensitive and activatable optical probes, and optical contrast agents based on nanoparticle and quantum dots, different radionuclide based nuclear imaging probes, and metal chelate based MRI probes [7–24]. This review will focus on the current status and use of these targeted molecular probes based on peptides, antibodies, small molecules, nanoparticles, affinity ligand based nuclear imaging probes, metal chelate based MRI probes and dual labeled probes. Furthermore, how these imaging agents can be used for optical/nuclear or multi-modal imaging platform will be discussed. Special attention will be given to optical, nuclear and MRI probes and their respective imaging techniques. Moreover, we will cover the recent development of multi-modal probes associated with nanoparticles, MRI agents, optical contrast agents and radionuclides and how these probes can be used synergistically in multi-modal imaging.
3. Imaging modalities
Three different non-invasive, in vivo imaging technologies (nuclear imaging, optical imaging, and magnetic resonance imaging) are evolving at the heart of molecular imaging for the better understanding of diseases.
3.1 Optical Imaging
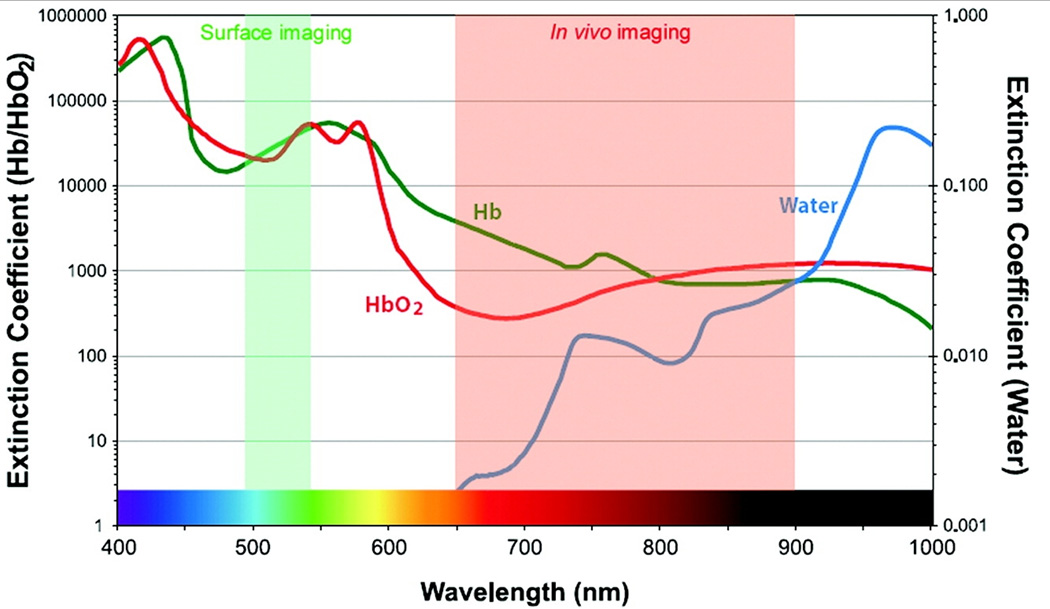
Optical imaging uses light from the visible and near-infrared regimes, and has the advantages over other modalities that include real time performance and sub-cellular resolution. The detectors are sensitive to a broad range of wavelengths, and can be used to image multiple probes in different bandwidths for multi-spectral imaging. As compare to whole body imaging systems, these instruments are more portable and less expensive. Optical imaging techniques with contrast agents have already been developed for in vitro and ex vivo applications in molecular and cellular biology (e.g., fluorescence microscopy). Contrast agents that use blue and or green excitation can achieve higher spatial resolution at the expense of less tissue penetration. However, these agents are also sensitive to tissue autofluorescence background and quenching from hemoglobin [Figure 1]. Thus, an optical window for in vivo imaging in the 665 to 900 nm range results in an optimal tradeoff among these parameters and in minimal absorption by hemoglobin and H2O. Several NIR fluorescent dyes have recently become available that can be coupled to affinity ligands (peptides, antibodies) or that are activatable [25]. The most common optical imaging techniques include confocal microscopy, two photon microscopy, fluorescence endoscopy, and fluorescence molecular tomography. The detailed instrumentation and working principle for these techniques is beyond the discussion of this review and can be found in a recent review [26].
Figure 1.
Extinction coefficient value of oxyhaemoglobin, deoxyhaemoglobin and water as a function of wavelength from visible to near –infrared wavelength. (Reproduced from 17 with permission)
3.2 Nuclear Imaging
Nuclear imaging instruments can visualize a very low concentration of radionuclide and provide quantitative information. However, scattering of radiation as it passes through the body results in a lower image resolution. The most common methods used for in vivo cancer imaging are positron emission tomography (PET) and single photon emission computed tomographic (SPECT). These techniques were first demonstrated in the 1970's as research tools. After 30 years, these whole body imaging techniques became mature enough for clinical use. In both techniques, an imaging agent is created by labeling an affinity ligand with a radioisotope. PET utilizes positron emitters (e.g. 11C, 18F), and detect γ rays that result from positron/electron annihilation, whereas SPECT directly images γ emitters (e.g. 125I, 111In, and 99mTc). Both techniques are valuable in molecular imaging and are capable of detecting minute amounts of radioactive tracers that vary in concentration from 10−10~10−12 molar. These levels introduce minimal perturbation of the biological systems being imaged. The sensitivity is relatively independent of the depth of the probe of interest. PET is more sensitive than SPECT by ~2 to 3 orders of magnitude, has better resolution, and offers superior probe quantification. On the other hand, SPECT is less expensive, has a broader choice in approved radionuclides, and can distinguish multiple emission energies simultaneously. Due to the last feature, SPECT has potential to distinguish co-administered tracers that differ in their targets and emission energies [27]. The use of PET and SPECT both can be limited by cost, exposes the patient to ionizing radiation, and results in lower temporal and spatial resolutions (1–30 min, 4–10 mm), compared to other imaging modalities. The use of ionizing radiation restricts the number of research studies that can be performed in a single patient. As a result the radioactivity dose is kept low (100–1000 Megabecquerel MBq), and is further limited if the tracer’s biodistribution results in accumulation in a particular organ(s).
3.3 Magnetic Resonance Imaging (MRI)
In addition, MRI has become increasingly popular for both experimental and clinical imaging due to its non-invasiveness and capability for producing three dimensional representations with high spatial and temporal resolution. Approximately 35% of all clinical MRI scans utilize contrast media, however a primary limitation of this imaging modality is the sensitivity of contrast agents and the requirement for high concentrations that vary from 0.1 to 0.6 mM [28]. Typically, MR images visualize anatomical structures based on their water content by measuring signals generated from protons in response to excitation by radio-waves that match the intrinsic frequency of processing protons. In molecular imaging, the strength of MR field is tailored to the detection of specific cells or even molecules inside anatomical structures at high resolution. Metals with magnetic moments (Gd3+, Mn2+, and Fe3+) are effective contrast agents for the MRI, discussed later in section 6.
3.4 Multi-modal imaging platform
Because each imaging modality has its own distinct advantage and limitations, combining a second imaging modality may provide better results and help overcome the limitations of the first modality. For example, the combined use of computed tomography (CT) and positron emission tomography (PET) is a successful example of multi-modal imaging. CT provides high-resolution anatomical detail to register the functional information provided by PET [29]. Currently there are very few examples of multi-modal imaging probes that can be detected by more than one technique: however, dual agents for detection by both radionuclide and optical imaging [30–31], or magnetic resonance (MR) and optical imaging [32–34] have been demonstrated. Another approach from optical imaging has been successfully applied in vivo, and uses a multi-spectral imaging strategy by applying two or more optical agents that can be differentiated on the basis of their fluorescence emission [35–37]. Similarly, confocal microscopy has recently been coupled with conventional white light endoscopy, and autofluorescence bronchoscopy has been integrated with OCT [38–39]. We expect that other combinations of high-resolution and wide-field optical imaging methods will soon follow. These types of multi-modality systems may provide a number of distinct opportunities, including improving the ability to quantify and locate molecular processes and characterizing new imaging probes [40]. However, to date, technical and practical issues have made it challenging to combine and translate these techniques into the clinic.
4. Molecular Probes for Optical Imaging
Targeted imaging with optical probes consists of three components: 1) a signaling moiety for readout, 2) a carrier molecule for optimizing pharmacokinetics, and 3) an affinity ligand for binding to the target. Small molecules have better pharmacokinetics and faster clearance compared to large molecules. Other important criteria for designing a promising probe include: the inherent properties of tissue that generate contrast, selection of fluorescent dyes, size and type of linker, and the nature of the affinity ligand. Thus, the development of optical imaging probes has always been one of the central focuses of molecular imaging. In the pursuit for an accurate diagnosis of disease in an early stage and to evaluate the response to therapy, several strategies have been employed for developing novel imaging probes over the past two decades. For an imaging probe to be clinically useful, it should provide a high “target-to background” ratio to maximize the image contrast in vivo. The ideal imaging compound would exhibit high binding affinity and specific uptake and retention in the target, rapid clearance from non-target tissues, high stability and integrity in vivo, ease of preparation, and safety for clinical use. This section reviews key components of exogenous molecular probes including, organic fluorescence dyes, peptides, antibodies, small molecules, and summarizes the different targeting principles associated with the design of these probes.
4.1 Non-specific optical contrast agents
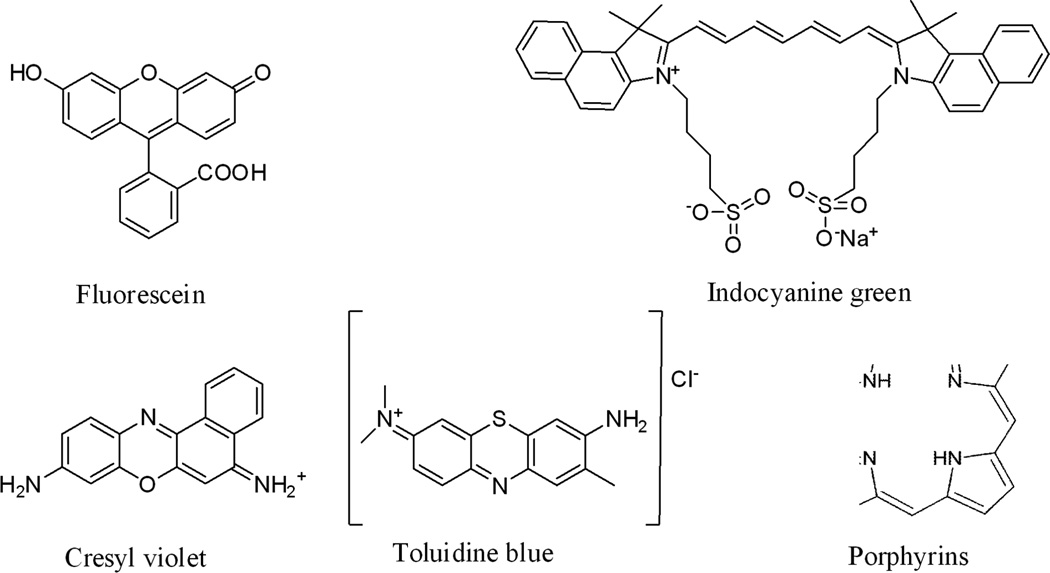
The first agents used in optical imaging include non-specific small molecules that have distinct absorbance or fluorescence properties or induce detectable changes in the presence of native tissue properties. These non-specific contrast agents enhance visualization of changes in cellular morphology that occur during cancer transformation. A range of dyes that have distinct optical properties include fluorescein, indocyanine green, cresyl violet, toluidine blue and Lugol’s iodine. In addition, porphyrins, chlorins, and rare earth metal chelates of terbium and europium have been used, shown in Fig. 2. These dyes are currently being used for research purposes in the clinic. These non-specific imaging agents can produce intra- or extra-cellular localization based on their size or charge distribution. Because of their low molecular weights (≤1 kD), these small molecules can be delivered efficiently via either topical or intravenous routes. However, their use can be limited by relatively high levels of non-specific background. Several studies with ICG dyes have been conducted and reviewed [41]. In a recent study, Kusano et.al. found high false positives (~30%) with injection of 2 ml ICG dye in to the subserosa around the tumor and caused unnecessary follow-up procedures [42].
Figure 2.
Chemical structure of the most common optical contrast agents used in clinic.
4.2 Target specific molecular probes
Image contrast and diagnostic accuracy can be improved significantly by using targeted contrast agents to enhance the signal from cancer specific molecules. Regardless of the imaging agent employed, targeting of disease specific markers is much more powerful for providing clear signals for diagnosis. Fluorescent dyes can be conjugated to specific substrates to form a molecular probe to visualize specific tumor targets. Several factors that need to be considered for selecting an appropriate target substrate include availability, feasibility of synthesis, low toxicity, specific target uptake and retention, rapid clearance from non-target tissues, high target binding affinity and high signal-to-noise ratio. Various conjugates have been reported that satisfy the most of the above properties and will be discussed below.
4.2.1 Conjugate with Peptide ligands
After the discovery of several specific peptide receptors in various cancer types, peptide analogs with good stability, receptor binding properties, and pharmacokinetic behavior were introduced for imaging neuroendocrine tumors, adenocarcinomas, lymphomas, and melanomas. Various optical contrast agents have been used for labeling such as visible and NIR dyes. However, recent efforts have focused on cyanine based NIR dyes for optical imaging, as they provide deeper tissue penetration [43–46]. In this section, we will focus on peptide-based fluorescence probes and their use for targeted imaging in cancer.
Peptides can be labeled with a variety of fluorophores that have different excitation and emission wavelengths. Fluorophores in the visible range, which exhibit emission between 400 and 600 nm such as 7-amino-4-methylcoumarin (AMC), fluorescein isothiocyanate (FITC), fluorescein carboxylic acid, and 5-carboxytetramethylrhodamine (TAMRA) are generally used for imaging with the best spatial resolution at the expense of tissue penetration. Tissue has significantly less autofluorescence at longer wavelengths in the near-infrared (NIR) region (650–900 nm) [47]. Chemical structures and optical properties of most NIR dyes are similar to that of indocyanine green, a FDA-approved tricarbocyanine dye commonly used as an angiographic agent. To date, a number of NIR dyes have been reported, and their reactive intermediates for peptide bioconjugation are commercially available. Examples include the family of Cy dyes from GE Healthcare, Alexa Fluor dyes from Invitrogen, IRdye dyes from Li-COR Bioscience, SRfluor dyes from Molecular Targeting Technologies, HyLyte fluor from Anaspec, and CF633 from Biotium. Many biologically active peptide analogues reported in the last decade have been labeled with NIR and visible fluorophores.
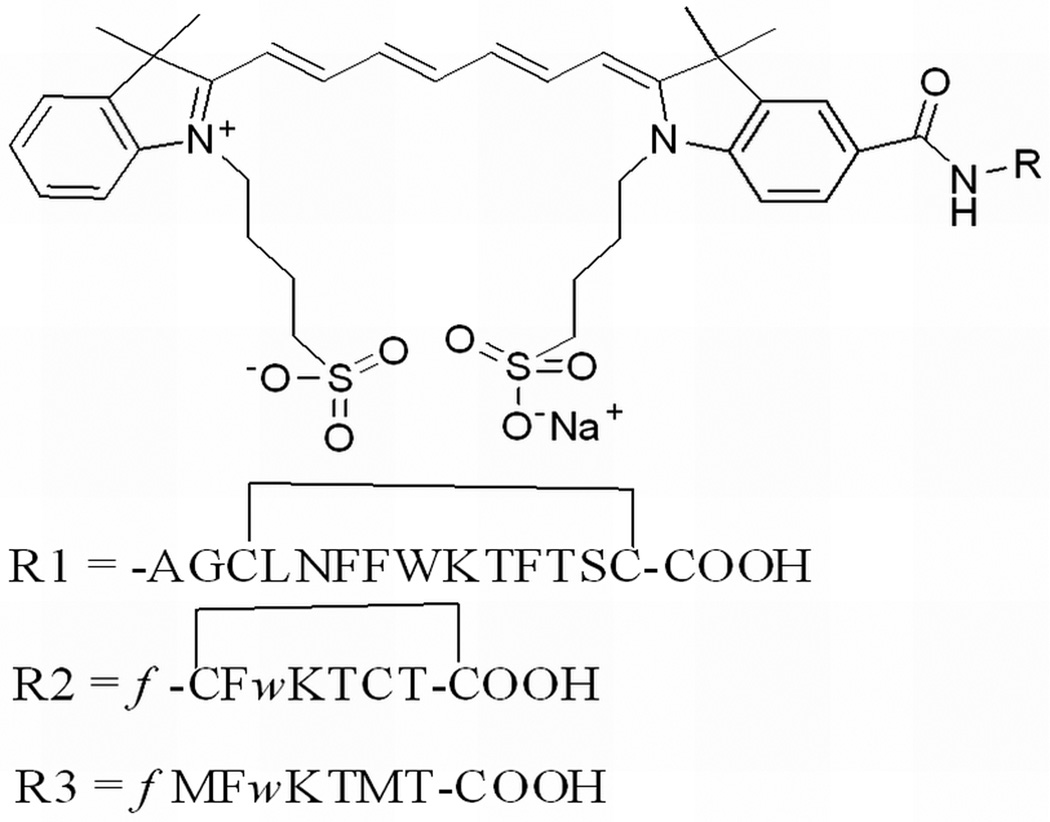
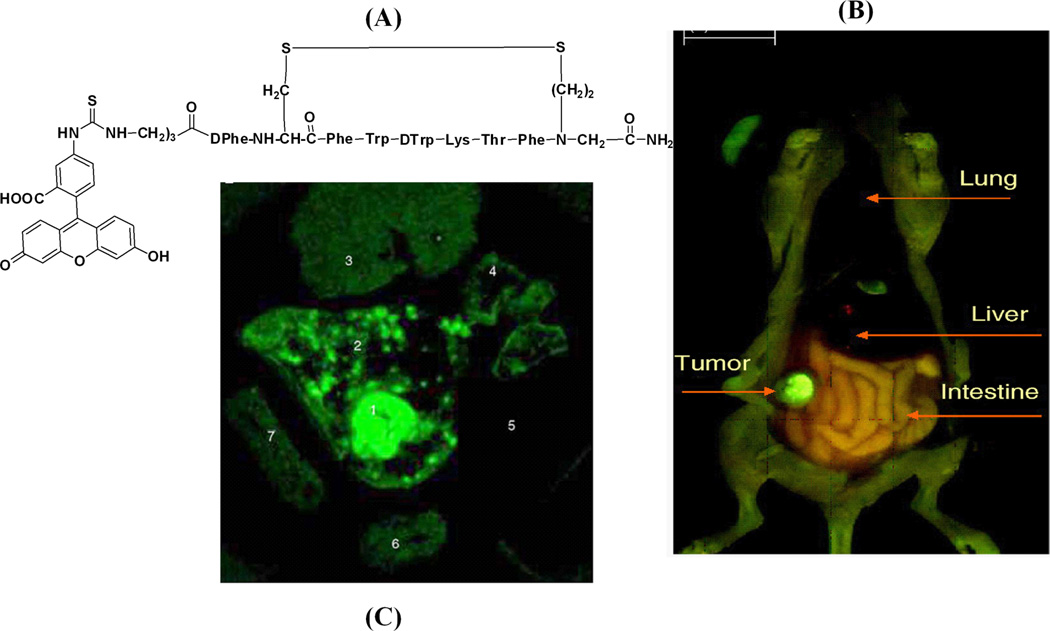
In vivo diagnostic use of a NIR-dye conjugate consisting of indotricarbocyanie (ITCC) dye and octreotate for tumor imaging has been reported, as shown in Fig. 3. The ITCC-octreotate conjugate exhibits fast receptor binding properties in SSTr-2-overexpressing RIN38/SSTr-2 cells and provided 3-fold higher tumor fluorescence in mice bearing RIN38/SSTr-2 tumors from 3 to 24 hrs after intravenous injection [48]. Different somatostatin analogues have also been labeled with fluorescein and rhodamine dyes and tested in SSTr-positive NCI-H69 human SCLC tumors and HT-29 colon tumor-bearing mice, respectively [49–50]. This result demonstrates for the first time that human SCLC can be specifically targeted with high selectivity by a fluorescent bioconjugate of somatostatin analog, providing high target to non-target (9~90) ratios, as shown in Fig. 4.
Figure 3.
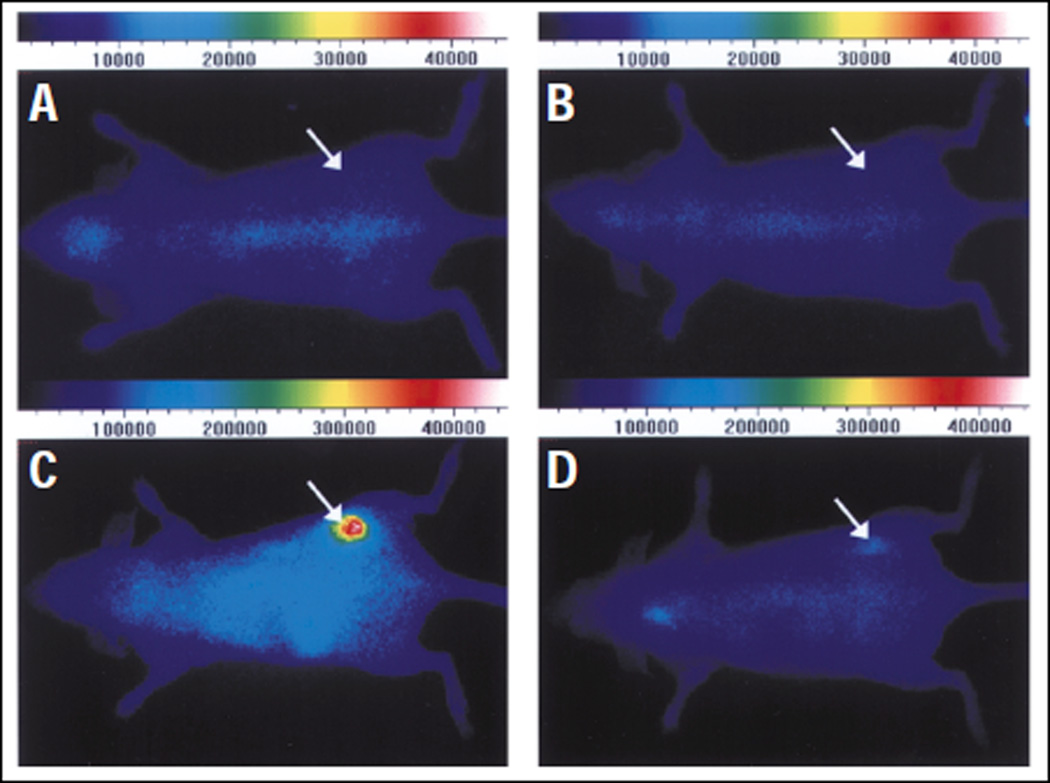
Chemical structure of ITCC conjugated Somatostatin (R1), Octreotate (R2) and M2M7 (R3) left; and in vivo fluorescence images with RIN38/SSTR2 tumor-bearing nude mice before the intravenous injection R2 (A) and R3 (B), top of the right panel. The bottom panel images were acquired after 6 hr. of intravenous injection of R2 (C) and R3 (D) at a dose of 0.02 µmol/kg body weight. (Reprinted with the permission of 48)
Figure 4.
FITC conjugated peptide probe (a); Total body fluorescence image of a surgically opened mouse with subcutaneously located H69 tumor at 24 h post-administration of FITC-conjugate peptide (b) and Spectrally resolved fluorescence of excised tumor and normal organs (1) fluorescence-brightfield images of H69 tumor nodule (2) skin, (3) liver, (4) lung, (5) pancreas, (6) kidney, and (7) spleen at 24 h post-administration of FITC-conjugate peptide. (Reprinted with the permission from 49)
Examples of molecular probes based on a peptide platform include bombesin conjugated to Alexa Fluor 680 (7–14) via a Gly-Gly-Gly linker. This probe was synthesized for targeted gastrin-releasing peptide receptor (GRPr) imaging and demonstrated specific GRPr targeting ability in vitro and in mice bearing T-47D breast cancer cells [51]. In addition, an enormous number of optical probes associated with angiogenesis-specific targets have been developed utilizing RGD peptide analogues. Chen et al., developed a Cy5.5 conjugated c(RGDyK) via an amide bond to the ε-amino group of the lysine residue and used this probe for imaging integrin αvβ3-positive U87MG tumor xenografts in mice [52]. In a separate study conducted by Cheng and Wu et. al., Cy5.5- or Cy7-conjugated mono-, di-, and tetrameric RGD peptides were developed, and demonstrated increased receptor binding affinity compared to those of their monomeric counterpart [53–54].
Furthermore, phage display is a high-throughput method developed using recombinant DNA technology to generate a library of clones that bind preferentially to the cell surface targets [55]. Peptides have been selected using this technique against a secreted protein acidic and rich in cystein (SPARC) for invasive cancer [56] and vascular cell adhesion molecule-1 (VCAM-1) for inflammatory endothelium [57]. Dye-labeled phage clones exhibited excellent in vivo targeting ability in tumors and VCAM-1 expression vessels, suggesting that fluorophore-based phage clones can be used as in vivo imaging probes.
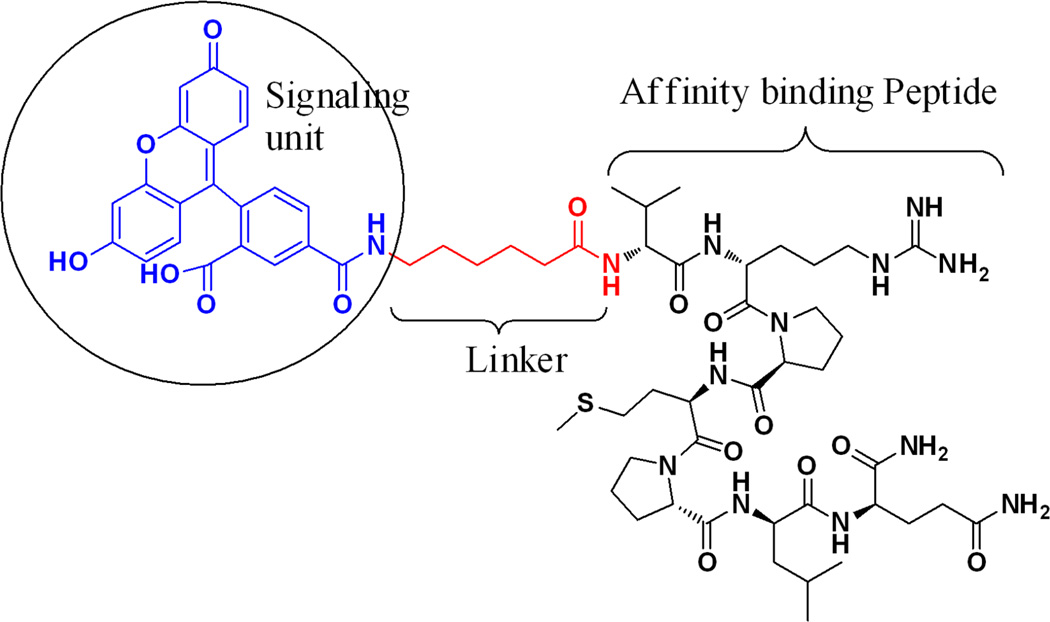
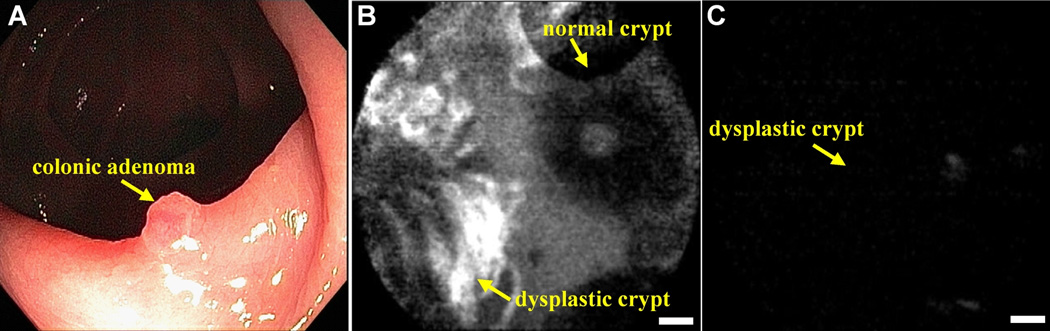
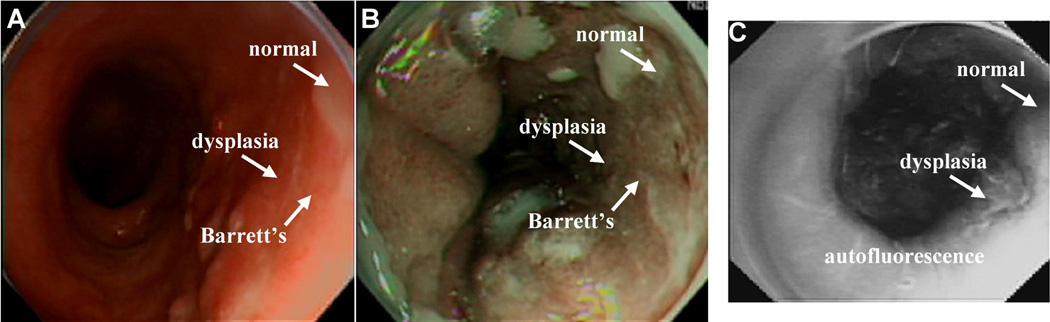
Similarly, we have successfully used this technique to identify peptides that bind preferentially to dysplastic rather than to normal colonic mucosa using a confocal fluorescence microendoscope to image preferential binding in vivo [58]. The human colonic adenoma-specific peptide VRPMPLQ was conjugated to fluorescein via an amino-hexanoic acid linker for clinical imaging, as shown in Fig. 5. Using a similar selection strategy for dysplastic esophageal mucosa, the phage with the peptide sequence ASYNYDA was identified and found to bind specifically to regions of high-grade dysplasia, as shown in Fig. 6. Thus, fluorophore-conjugated peptides represent a powerful tool for targeted imaging in the clinic and a potential alternative to other imaging modalities in pre-clinical settings.
Figure 5.
Chemical structure of phage derived peptide with sequence VRPMPLQ conjugated with fluorescein via aminohexanoic linker (top panel) and in vivo validation to colonic adenoma by confocal endomicroscopy (bottom panel). (A) Conventional white light endoscopic image of colonic adenoma. (B) In vivo confocal image following topical application and incubation with peptide, which shows binding to dysplastic (left half) and no binding to normal (right half) crypts. (C) Confocal image with control peptide (scrambled), sequence QLMRPPV, shows no binding to adenoma (scale bar 20 mm) (Reprinted from 58 with permission).
Figure 6.
In vivo localization of peptide binding to high-grade dysplasia in Barrett’s esophagus on wide-area endoscopy. (A) A conventional white light endoscopic image of Barrett’s esophagus shows no evidence of premalignant lesions. (B) NBI of the same region shows improved contrast, highlighting intestinal metaplasia but not dysplasia. (C) In vivo fluorescence image following topical administration and incubation with phage born affinity peptide, sequence ASYNYDA, which binds preferentially to high-grade dysplasia.
4.2.2 Conjugate with antibodies
Targeted imaging has also been demonstrated with antibodies and their modified analogues, including antibody fragments, diabodies and minibodies. The strategy of coupling cyanine dye labels to affinity-maturated single-chain antibody fragments directed against an angiogenesis-specific oncofetal isoform of fibronectin (ED-B fibronectin) for in vivo imaging was demonstrated by Folli et al. [59] and Ballou et al. [60]. When Cy7 or Cy5.5-labeled antibody fragments were injected, the expression of ED-B-fibronectin was visualized in tumour-bearing mice, in a mouse atherosclerosis model, and in angiogenesis induced in the cornea of rabbits, demonstrating the relevance of ED-B-fibronectin as a marker of angiogenesis [61–63]. In separate studies conducted by various independent research groups, cyanine dyes conjugated to antibody- and protein-based ligands with epidermal growth factor EGF [64], endostatin [65], and an IgM antibody targeting the endothelial expression of glycoproteins were used for the optical imaging of inflammation and lymph nodes [66].
ICG dye has been approved by US FDA for intravenous use to measure cardiac output, assess hepatic function, and visualize ocular vessels. This dye has been conjugated with antibodies for targeting the detection of cancer in the digestive tract. Reactive derivatives of ICG including ICG-N-hydroxysulfosuccinimide ester (ICG-sulfo-OSu) and 3-ICG-acyl-1, 3-thiazolidine-2-thione (ICG-ATT) have been developed and labeled with anti-CEA and anti-MUC1. These conjugated compounds exhibit small shifts in the excitation and emission wavelengths relative to pure ICG, and have been used to demonstrate the proof of principle for targeted endoscopic imaging [67]. Furthermore, monoclonal ICG-labeled mouse anti-CEA antibody has been used to target gastric cancer on biopsy specimens and imaged with near-infrared endoscopy. A conventional white light endoscopic image was collected first from freshly resected gastric mucosa, and then a near-infrared fluorescence image of the same specimen stained with ICG labeled anti-CEA antibody was acquired, revealing foci of cancer. In addition, specimens of normal gastric mucosa did not reveal antibody binding, and were used as controls, as shown in Fig. 7.
Figure 7.
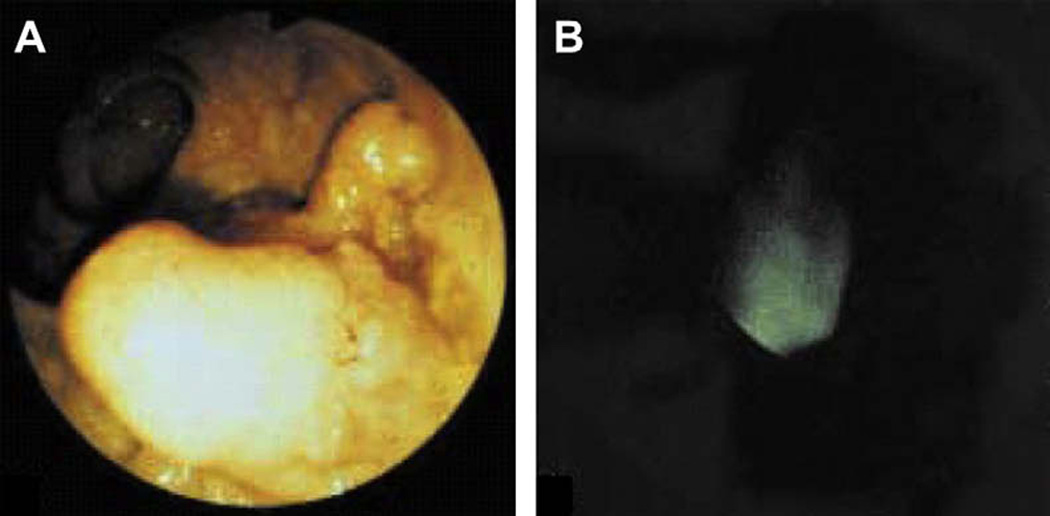
In vivo localization of anti-CEA antibody binding to colonic adenoma on fluorescence endoscopy. (A) A conventional white light endoscopic image of a colonic adenoma and (B) corresponding fluorescence image collected in vivo after topical administration and incubation with the dye- labeled anti-CEA antibody shows increased intensity at the site of the lesion. (From Keller, R.; Winde, G.; Terpe, H. J.; Foerster, E. C.; Domschke, W. Fluorescence Endoscopy Using a Fluorescein-labeled Monoclonal Antibody Against Carcinoembryonic Antigen in Patients with Colorectal Carcinoma and adenoma. Endoscopy 2002, 34, 801–807 with permission.)
In a recent study, Goetz et.al used a fluorescent-labeled whole antibody that targeted epidermal growth factor receptor (EGFR), and demonstrated binding of this antibody conjugate against cell lines with either low or high EGFR expression. This probe was also validated on xenografts grown subcutaneously in mice and on ex vivo human samples. Imaging was performed using a confocal laser endomicroscope with 488 nm excitation, and high-resolution images were collected from the resultant fluorescence in the 505- to 585-nm range. This study demonstrates that molecular imaging is feasible in vivo by targeting EGFR. [68]
4.2.3 Conjugate with small molecule ligands
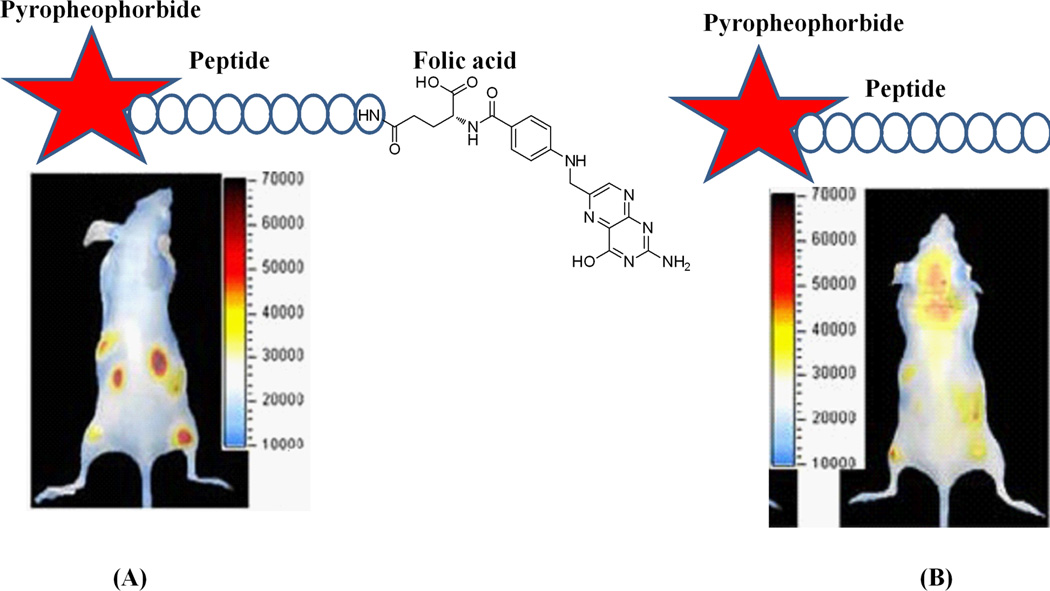
Unlike the non-specific dyes discussed in the section 4.1, targeted small molecules can be effective contrast agents because of their relative ease of delivery into tissue, ability to mediate specific molecular interactions, and rapid uptake into tumor cells. Several small molecule affinity ligands conjugated with fluorescence dye are shown in Fig. 8. Among these, folic acid, cobalmin, 2-[N-(7-nitrobenz-2- oxa-1, 3-diazol-4-yl) amino]-2-deoxy-D-glucose (NBDG; a fluorescent analog of FDG), bis-phosphonate pamidronate/alendronate are frequently used for targeted imaging. Folic acid is a promising ligand that has been employed as carrier molecule to target the abundant folate receptors on proliferating cells and activated macrophages. After conjugation of folic acid with the cyanine dye Cy5.5 or NIR2, uptake studies demonstrated promising imaging properties in cancer models [69–70] similar to that of peptide ligands and showed utility for the early detection of inflammatory disease in a rheumatoid arthritis model [71]. More recently, the PDT agent pyropheophorbide conjugated with a small peptide linker and folic acid has been developed for a folate mediated targeting probe which improves the efficiency of targeted delivery of NIR imaging and PDT agents, as shown in Fig. 9 [72].
Figure 8.
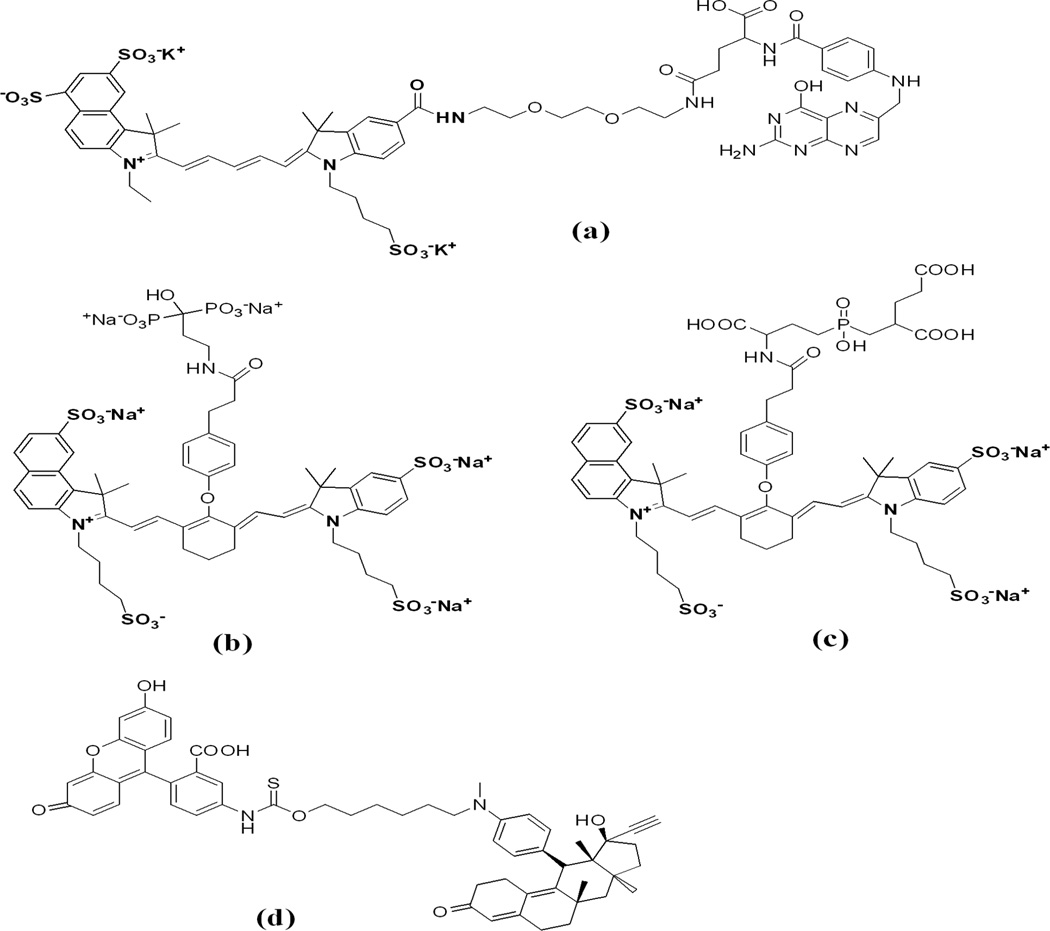
Chemical structures of fluorophore conjugates with small molecule ligands; (a) Cy5.5 dye conjugated with folic acid via PEG linker; (b) bone-targeted IRDye78 pamidronate Pam78; (c) IRDye78 conjugate with PSMA (Prostate Specific Membrane Specific) ligand GPI and (d) progesterone receptor antagonist mifepristone labeled with fluorescein isothiocyanate.
Figure 9.
In vivo distribution of intravenously injected Pyro-peptide-Folate (PPF, targeting Folate Receptor positive KB tumor) (A) and Pyro-peptide (PP, no targeting, B). Xenogen images of double tumor mice (HT 1080 tumor left, KB tumor right) injected with PPF (left) or PP (right) at 6.5 hours after injection shows higher accumulation of PPF in the KB tumor (Reprinted with the permission from 72).
4.3 Activatable or “smart” probes
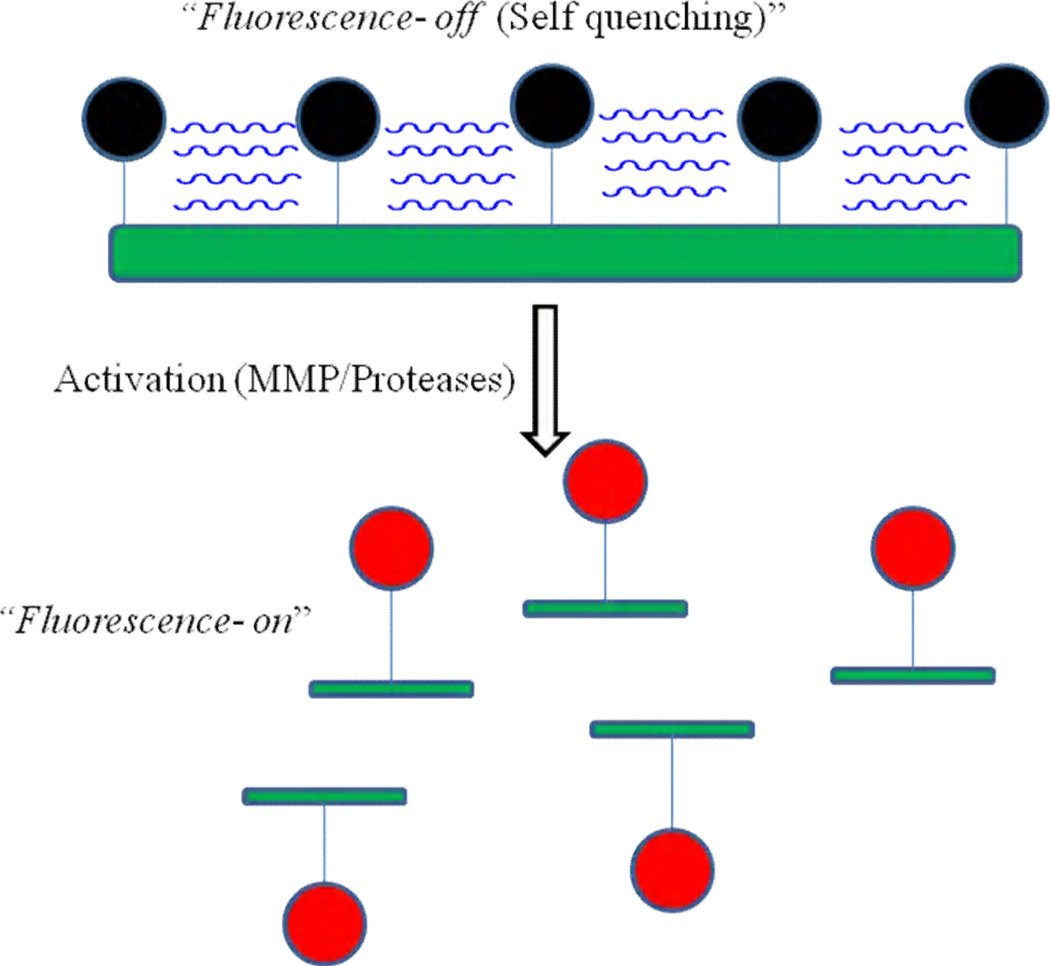
Activatable or “smart” probes are another class of imaging agent, which allows for fluorescence to be activated by the microenvironment. Fluorescence activation involves the conversion of a fluorophore from a quenched, non-emitting state into a free state where it is capable of emitting fluorescence upon excitation. Compared to the fluorophore-labeled peptide probes, an activatable probe is optically dark in its quenched state and becomes strongly fluorescent in vivo following enzymatic cleavage. The initial idea advanced by Weissleder et. al. for conjugation of the Cy5.5 dye to the backbone of poly (L-lysine)/poly (ethylene gylcol) graft polymer, and enabled the establishment of a multi-faceted molecular imaging platform [73]. Conjugation of the fluorophores either directly to or via cleavable peptide sequences in close proximity to each other causes strong fluorescence quenching. As illustrated in Fig. 10, enzymatic cleavage by cathepsins, MMPs and other proteolytic enzymes converts the probe from a quenched, non-detectable state to a fluorescence-emitting state by liberating single dye-loaded fragments either through backbone cleavage of the lysine-lysine amide bonds or cleavage at the peptide sequences.
Figure 10.
Schematic diagram of enzyme activated m-PEG Poly-lysine based probe. The initial proximity of the fluorochrome molecules to each other results in signal quenching (top) and after getting activated by enzyme, the probe will get activate and shows the signal (bottom).
A wide range of applications has been demonstrated with enzymatic degradation in many disease models, including cancer, atherosclerosis, rheumatoid arthritis, and thrombosis using this simple operating principle based on the self quenching of dyes. Self quenching is generally not as efficient as the use of custom designed molecular quenchers, such as QSY7 and dabcyl group. Ogawa et.al demonstrated a new class of activation mechanism using the TAMRA (fluorophore)-QSY7 (quencher) pair. This design is based on the conjugation of these fluorophore-quencher pair to either avidin (targeting the D-galactose receptor) or to trastuzumab (an FDA approved monoclonal antibody against the human epithelial growth factor receptor type2 (HER2/neu)), and its performance in mouse models of cancer has been demonstrated. Two probes, TAMRA-QSY7 conjugated avidin (Av-TM-Q7) and trastuzumab (Traz-TM-Q7) were synthesized. Both demonstrated better performance than similar self-quenching probes. In vitro fluorescence microscopic studies of SHIN3 and NIH/3T3/HER2+ cells demonstrated that Av-TM-Q7 and Traz-TM-Q7 produced high intra-cellular fluorescence signals as compare to the negative control conjugate Dac-Tm-Q7. In vivo imaging with Av-TM-Q7 and Traz-TM-Q7 in mice has demonstrated detection of small tumors, demonstrating proof of principle for this design [74].
Recently, Urano et.al developed a novel approach for activatable probes which utilizes the photo-induced electron transfer (PeT) mechanism for fluorophore activation [75]. At physiologic pH (~7.4), a non-protonated N,N-dialkylated aniline moiety is able to virtually eliminate fluorescence emission from the independent 2,6-dicarboxyethyl-1,3,5,6-tetramethyl boron-dipyrromethane (BODIPY) dye through the PeT mechanism. However, at a low pH (pH ~5–6) found in lysosomes, protonation hampers PeT, resulting in a 300 fold increase in photon emission. After conjugation to a trastuzumab antibody, this probe is able to image HER-2+ cells via internalization. Because the release of fluorescence is pH dependent, this probe demonstrates reversibility (or “deactivation”) if it is ejected from the tumor cell into the more neutral extra-cellular environment, or if the tumor cell becomes non-viable. This approach is potentially advantageous to other protease/enzyme degradation mechanisms where an activated agent that leaks away from the activation site will produce non-specific signal. The pH activated probes will deactivate if they escape their target environment, and not release non-specific fluorescence.
4.4 Optical probes based on Nanoparticles
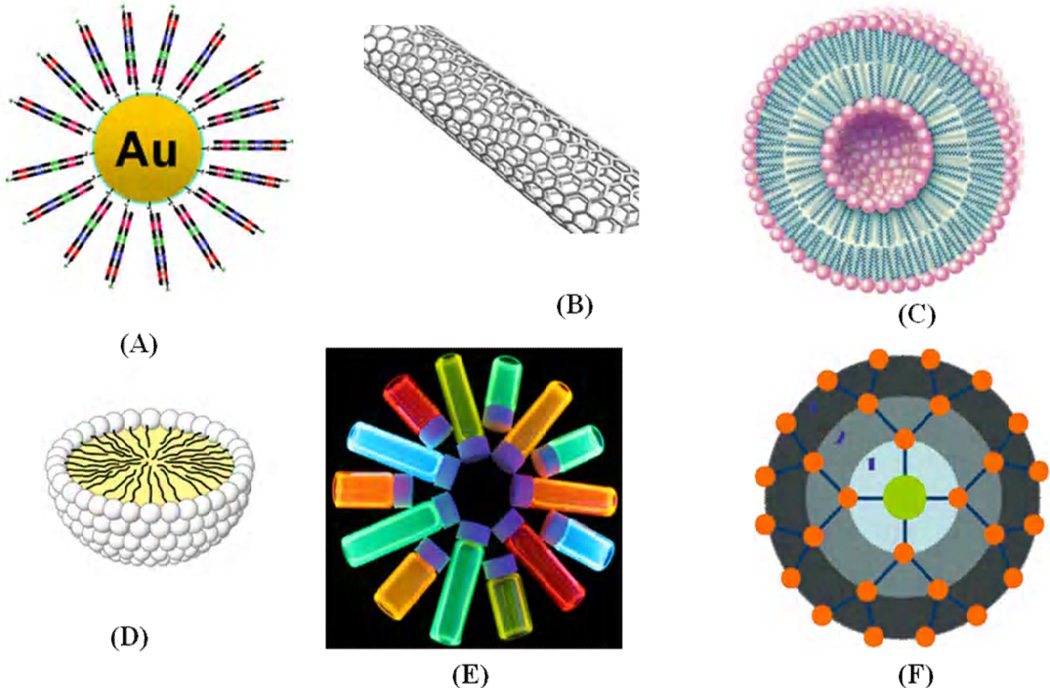
Nanoparticles are promising agents for molecular imaging because they provide higher signal intensity than organic dyes, allowing for detection of targets that are expressed at lower levels [76–77]. In addition, nanoparticles have desirable spectral properties such as broad and narrow excitation bands and tunable fluorescence emission spectra that can be used for multi-spectral imaging. Due to their relatively large surface areas, they are ideal for efficient modification, and can be used as multiple probes that enable multi-valent binding to one or more targets, increasing the affinity of individual probes. There are several promising examples of nanosized materials that have been thoroughly investigated, including quantum dots (QDs), polymeric nanoparticles, carbon nanotubes, and gold nanoparticles, as shown in Fig. 11. Over the past decade, there have been significant advancements in the field of nanoparticle-based molecular imaging [78–80]. An early demonstration of in vivo targeted imaging was shown by Gao et al. in a mouse model using CdSe-ZnS quantum dots with a co-polymer functionalized surface conjugated to an antibody targeting the prostate specific membrane antigen. In vivo targeting studies of human prostate cancer growing in nude mice indicated that the QD probes accumulate at tumors by enhanced permeability, retention in tumor sites, and antibody binding to cancer-specific cell surface targets. Multi-color fluorescence imaging of cancer cells in vivo was achieved by subcutaneous and systemic injection of QD-tagged cancer cells [81]. Similarly, quantum dot based imaging probes associated with the RGD peptide (QD705-RGD) as a model targeting system was suggested by Cai et al. in an in vivo murine xenograft study targeting αvβ3, an integrin which is commonly expressed in angiogenesis and metastasis [82]. While in vivo imaging of QDs has been shown to be highly efficacious due to their superior brightness and photostability for monitoring cancer development at the early stage in animals, their future clinical use as imaging agents is questionable, as long-term toxic effects generated by traces of heavy metals have not yet been addressed. Therefore, metal-free systems would be advantageous, provided that they exhibit comparable photo-optical properties.
Figure 11.
Various types of nonmaterial which were used as contrast agents for targeted imaging (a) gold nanoparticles; (b) carbon nanotube; (c) liposome; (d) micelle; (e) quantum dots and (f) dendrimer.
5. Molecular probes for nuclear imaging
In clinical research, nuclear imaging methods are widely used because of their high sensitivity and are compatible with injection of radio-labeled probes in minute quantities. PET and SPECT are the most sensitive nuclear imaging techniques. These modalities are sensitive to molecular probes in the human body in the picomolar range, and can visualize many interactions between physiological targets and affinity ligands. Unlike fluorophores in optical imaging, there are various kinds of radionuclides that can be conjugated to affinity ligands. To develop a new radio-labeled probe, the primary concern is the isotope half-life which must be long enough to complete synthesis and to be administered to the patient. Ideally, the biologic half life of the final compound will have a sufficient duration but no longer than absolutely needed. For example, antibodies have long clearance times and longer lived radioisotopes such as 131I, 111In, 186Re, 67Ga, 177Lu for SPECT, and 124I, 64Cu, 86Y for PET are preferred.
On the other hand, small molecules that are cleared rapidly may require labeling with shorter lived isotopes such as 11C and 18F. Another concern for selecting the ideal radionuclide is rapid synthesis of the compound. In addition, to avoid the exposure from radiation, the reaction should be done in a “hot-cell”, a radio-protected closed box equipped with an automatic synthesis system. Thus, the simple and fast reactions are always highly desirable for short half-life radionuclides, a task that is often challenging for radiochemists. For PET, a cyclotron can be used to produce the radioisotopes (radioactive chemical elements) which are used to synthesize the radiopharmaceuticals (the compounds which are used to make the functional images of the body). The cyclotron is an accelerator of subatomic particles. Protons are produced in large quantities and are accelerated along a circular orbit inside a chamber controlled by powerful alternating electromagnetic fields. Thus, the particles gain energy and are smashed against a target at nearly the speed of light. The atoms of a substance placed in this target are transformed into radioactive, unstable isotopes by means of a nuclear reaction. There are many radioactive isotopes that can be produced in the cyclotron, including 11C, 13N, 15O and 18F which are commonly used for PET scanning and their half lives vary from a couple minutes to ~ 2 hr.
Various kinds of nuclear imaging probes have been previously presented, and some of them have been successfully applied for clinical research. The glucose analog, 18F-fluoro-2-deoxy-D-glucose (18F-FDG), has been used extensively in molecular imaging, and was originally developed as a chemotherapeutic agent to target cells that depend on high levels of glycolysis. At therapeutic levels, FDG was found to toxic to the central nervous system and was no longer used clinically [83]. Because of the high sensitivity of PET, 18F-FDG, can be given at safe doses and provide a method for evaluating glucose utilization. Clinically, some success using 18F-FDG for estimating the metabolic activity of tumors was achieved, but the ability to distinguish tumor from benign conditions where upregulated glycolysis process occurs in normal and malignant organs was less promising [84–86].
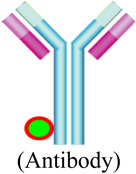
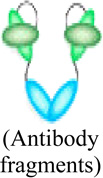
Conjugation of radionuclides to antibodies is another approach for developing targeted nuclear imaging probes. Due to their variety, several studies have been conducted to demonstrate pre-clinical applications of radio-labeled antibodies for identifying specific cellular targets. Radiolabeled antibodies and antibody derivatives provide the ideal platform for imaging tumor associated cell surface antigens, overcoming the limitations of less specific imaging methods. A number of engineered antibodies, fragments and derivatives have been developed such as 90Y conjugated to ibritumomab tiuxetan, a monoclonal antibody to CD20 (clinically approved for non-Hodgkins lymphoma), minibodies (~75 kDa) [87], diabodies (~50 kDa) [88], disulfide-stabilized and linear single chain variable fragments (scFv) (~25 kDa) [89], and affibodies (~7 kDa) [90–91]. These engineered antibody fragments have been demonstrated successfully in pre-clinical studies.
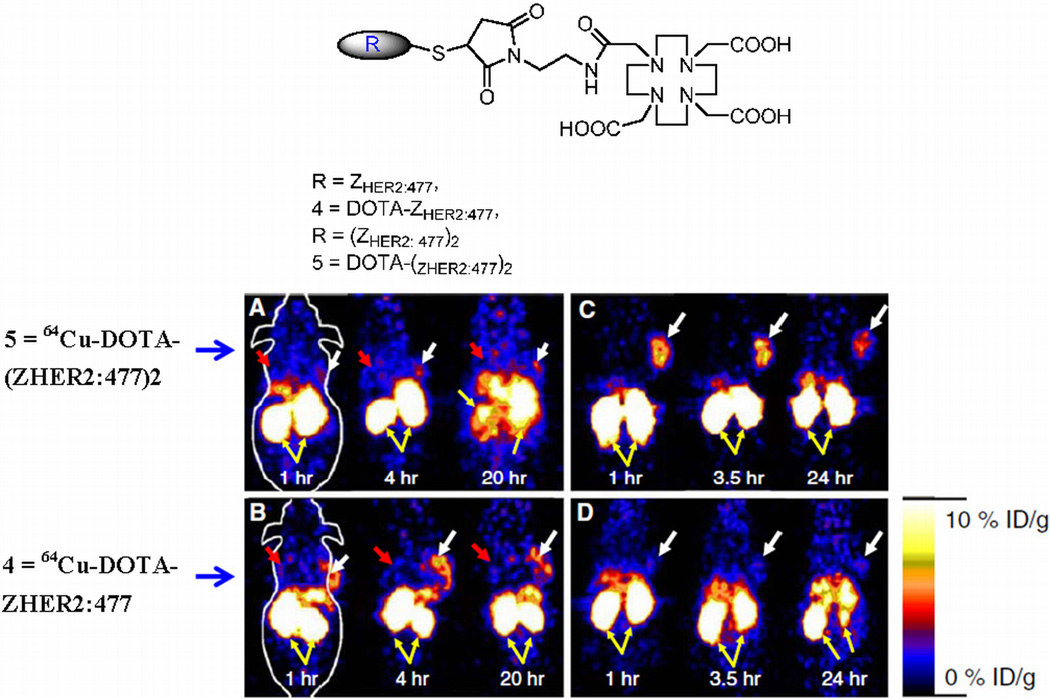
In a recent study by Cheng et al. human epidermal growth factor receptor type 2 (HER2)-binding affibody molecules in their monomeric and dimeric forms were site specifically modified with the maleimide-functionalized chelator, 1,4,7,10-tetraazacyclododecane-1,4,7-tris(acetic acid)-10-acetate mono (N-ethylmaleimide amide) (Mal-DOTA), radiolabeled with 64Cu and their imaging performance was further evaluated in SKOV3 tumor mice models. Biodistribution experiments showed that tumor uptake values of 64Cu-DOTA-labeled monomeric and dimeric Her-2 antibodies were 6.12±1.44% and 1.46±0.50% ID/g, respectively, in nude mice (n=3 each) at 4 hrs post injection. Moreover, 64Cu-labeled monomers exhibited significantly higher tumor/blood ratio than that of radiolabeled dimeric counterpart at all time points examined in this study. As shown in Fig. 12, MicroPET imaging of 64Cu-DOTA-labeled monomeric Her-2 in SKOV3 tumor mice clearly showed specific tumor localization [92]
Figure 12.
Chemical structures of the anti-HER2 affibody molecules (top panel). Bottom pannel are the decay corrected coronal microPET images of nu/nu mice bearing SKOV3 (indicated by white arrows) and MDA-MB-435 tumor (indicated by red arrows) at 1, 4, and 20 h after tail vein injection of 64Cu-DOTA-(ZHER2:477)2 (a) and 64Cu-DOTAZHER2: 477 (b). Decay corrected coronal microPET images of SKOV3 bearing mice which were pretreated with PBS (c) or 300µg of Herceptin (d) 48 h before probe administration at 1, 3.5, and 24 h after tail vein injection of 64Cu-DOTA-ZHER2:477. Yellow arrows indicate location of kidneys (reprinted with the permission from 92).
Another strategy for performing targeted imaging with a nuclear platform is to use radiolabeled analogues of naturally occurring peptides that target endogenous receptors that are differentially over expressed in tumors [93–94]. Most peptide probes are designed to perform a regulatory function and mimic naturally occurring peptides with different sizes ranging from a few to tens of amino acids. These peptides play an important role in modulating the physiological conditions from their specific and high-affinity peptide binding receptors. Many of these peptide-binding receptors are highly over expressed in many cancer types [95]. The growing evidence for peptide-binding receptors over expressed in specific tumors has stimulated interest in the development of peptide-based probes by using radiolabeling techniques [96–97]. Recent advances in combinatorial peptide chemistry and techniques of phage display have led to the development of well established strategies for the design of receptor-specific small peptides [98–99].
Various radionuclides, including 99mTc, 123I, 111In, 18F, 64Cu, and 68Ga, have been used as labels for peptides via a chelating moiety or a prosthetic group. The peptide can be labeled with the appropriate radiometals using the chelating group covalently conjugated to the peptide or by direct labeling if the functional groups of the peptides are able to act as metal coordinators [100]. The most widely used agents include branched chelators such as di-ethylene tri-amine penta-acetic acid (DTPA), 1, 4, 7, 10-tetra-azacyclododecane-1, 4, 7, 10-tetraacetic acid (DOTA) and their analogues. These chelating agents utilize carboxylate and amine groups to form stable complexes with radioactive metals such as 111In, 64Cu, 68Ga, 86Y, 90Y, and 177Lu. Chelating agents, such as di-amine dithiols, activated mercapto acetyl-glycyl-glycyl-gylcine (MAG3), and hydrazidonicotinamide (HYNIC), are able to chelate metals like 99mTc and 186Re. Instead of using chelating agents, a prosthetic group such as N-succinimidyl-4-18F-fluorobenzoate (18F-SFB) is necessary for labeling peptides with 18F.
Utilizing these radionuclides, various peptides have been developed including the FDA approved 111In-Octreotide and 99mTc-Depreotide. 111In-octreotide is an 8-mer peptide which was introduced in the mid-1990s. This peptide residue binds to somatostatin subtype-2 receptor which is over expressed in neuroendocrine tumors, making it an attractive choice for imaging. Depreotide (a somatostatin analog) is another small synthetic 10-amino-acid peptide that complexes with 99mTc and binds with high affinity to subtypes 2, 3, and 5 of somatostatin receptors that are over expressed on small cell lung cancer (SCLC) cells and other pulmonary malignancies, as well as non–small cell lung cancer [101–102]. This compound was approved by the FDA in 2002 and has emerged as a useful non-invasive tool for SPECT imaging to evaluate indeterminate solitary pulmonary nodules and to accurately stage lung cancer. These clinically approved 111In labeled DTPA octreotide (OctreoScan) and 99mTc labeled depreotide have proven to be a successful and versatile molecular imaging agent [103–104].
Similarly, various other hormone analogues in different stages of pre-clinical and clinical development include bombesin to target gastrin releasing peptide receptor, vasoactive intestinal peptide (VIP) to target the VIP receptor, and RGD peptide to target α5β3 integrin [105]. Other peptides under pre-clinical investigation include epidermal growth factor (EGF), glutathione (GSH), and a laminin-ligand (YIGSR). All of the efforts and successes in this field offer promise for localizing different tumors and metastases and for producing satisfactory peptide radiopharmaceuticals for diagnostic and therapeutic use.
Another strategy for developing molecular probes for nuclear imaging includes the labeling of multi-functional nanoparticles such as iron oxide, perfluorocarbon, nanotube, quantum dot, micelle, liposome and dendrimer with suitable radionuclides [106].
6. Molecular probes for MRI
Magnetic resonance imaging (MRI) is a widely used non-invasive imaging modality. Because of rapid advances in this technology, the temporal and spatial resolution that can be achieved has improved considerably, and the popularity of MRI has grown significantly in recent decades. Recently, much attention has been given to MRI contrast agents, including both low molecular weight agents and macromolecules, for their ability to improve the image quality of MRI. The two major categories of magnetic resonance probes are paramagnetic and super paramagnetic agents. Paramagnetic metal complexes, such as the gadolinium (III) ion-DTPA (Gd3+-DTPA) complex, are widely used in clinical diagnosis. Such gadolinium complexes enhance T1-weighted images by shortening the T1-relaxation time in water. The chelating moiety prevents the paramagnetic lanthanide ion from becoming toxic. Paramagnetic agents generate magnetic moments which speed up the relaxation time of protons in water following a radiofrequency pulse, resulting the shorter T1 and T2 relaxation times and increasing the signal. Super paramagnetic agents consist of an iron oxide core or a Fe/Mn composite metal core covered in a polymer matrix to prevent aggregation, and they form a significantly larger magnetic moment than that for the paramagnetic agents. Generally, these agents shorten the T2 relaxation times. However, a newer generation of smaller super paramagnetic agents has been reported to affect T1 as well [107]. Both of these agents function primarily through a perfusion mediated process distributed throughout the intravascular and interstitial space. Due to their high vascularity and inefficient lymphatic drainage, they can be used for cancer imaging by localizing tumor. This approach provides a means to monitor the tumor response to therapy.
IONPs (iron oxide nanoparticles) are one of the most studied MRI contrast agents because of their superior magnetic properties, ease of modification, and biocompatibility. Currently, several IONP formulations have been approved by the FDA, and are used in the clinic for visualizing the bowel, liver, spleen, and lymph node. However, low sensitivity is still a major hurdle that needs to be overcome. Several strategies have been pursued for increasing sensitivity, including conjugation to peptides and antibodies. This approach will not only increase the sensitivity but will also perform targeted delivery of the contrast agents. So far, this approach has not been widely adopted [108–109]. For the purpose of delivering higher magnetic payloads, Colpitts et al. conjugated multiple magnetic nanoparticles to a targeted carrier molecule, such as a peptide, dendrimer, and liposome. However, this conjugation step alters the pharmacokinetics of the probe as well as the magnetic effects of the metal nanoparticles [110]. Several advances in the use of polymeric micelles as MRI contrast agents have recently been reported [111–114], but so far significant enhancements of MR images with solid tumors through accumulation of the polymeric micelles have not been obtained.
7. Dual labeled probe for multi-modal imaging
Multi-modality imaging has the potential to overcome the limitations of a single modality by integrating the advantages of different approaches. This section discusses multi-modal probes that have been designed for one imaging modality but can also function in another imaging platform. The number of probes that have been developed in this growing field so far is limited. However, we expect that these probes will be useful for cross validating imaging results. The first multi-modality probes were developed [115–117] for imaging in the optical/MRI platform by combining fluorescent dyes with iron oxide nanoparticles. By taking advantage of the dual labels, in vivo MRI and optical imaging [118–120] can be performed with target-specific delivery via molecular vehicles attached to the coating of the multi-modal particle. Other systems include a mono-molecular agent consisting of a heptamethine carbocyanine and a 111In-DOTA chelate for optical/nuclear and a chlorophyll-a analogues conjugated with amino benzyl-DTPA for photodynamic therapy agent for MRI. [121].
Kobayashi et al. synthesized nanoprobes with multi-modal and multi-color applications, which employ a polyamidoamine dendrimer platform (6-PAMAM) linked to both radionuclides and optical probes, permitting dual-modality scintigraphic and five-color near-infrared optical imaging of lymphatics in the head and neck of mice. Radionuclide imaging provided semi-quantitative information and optical imaging provided qualitative information for each of the five lymphatic basins with excellent spatial resolution, suggesting that future application to sentinel node imaging is promising. Ogawa et al. suggested another approach for developing multi-modal probes by combining activatable optical probes with radioactive probes for targeted imaging of Her1 and Her2 tumor bearing mice. ICG dye and 111In were selected for the labeling of monoclonal antibodies panitumumab and trastuzumab in Her1 and Her2 bearing tumor mice. Both conjugates were internalized into the cells and showed bright fluorescence signals only in the target cells on optical imaging, and similar results were obtained with a dual labeled probe in both imaging platforms without affecting the pharmacokinetic properties [122]. Another distinct approach suggested by Liu et. al. for imaging with the SPECT/MRI platform was based on the conjugation of Fe3O4 nanoparticles and 125I with a monoclonal antibody via a PEG linker. The sensitive γ-imaging results based on the covalently attached 125I provide additional information on biodistribution of the dual-modality probe, suggesting that this may be a more sensitive approach to evaluate the in vivo behavior of nanoparticles-based molecular probes [123].
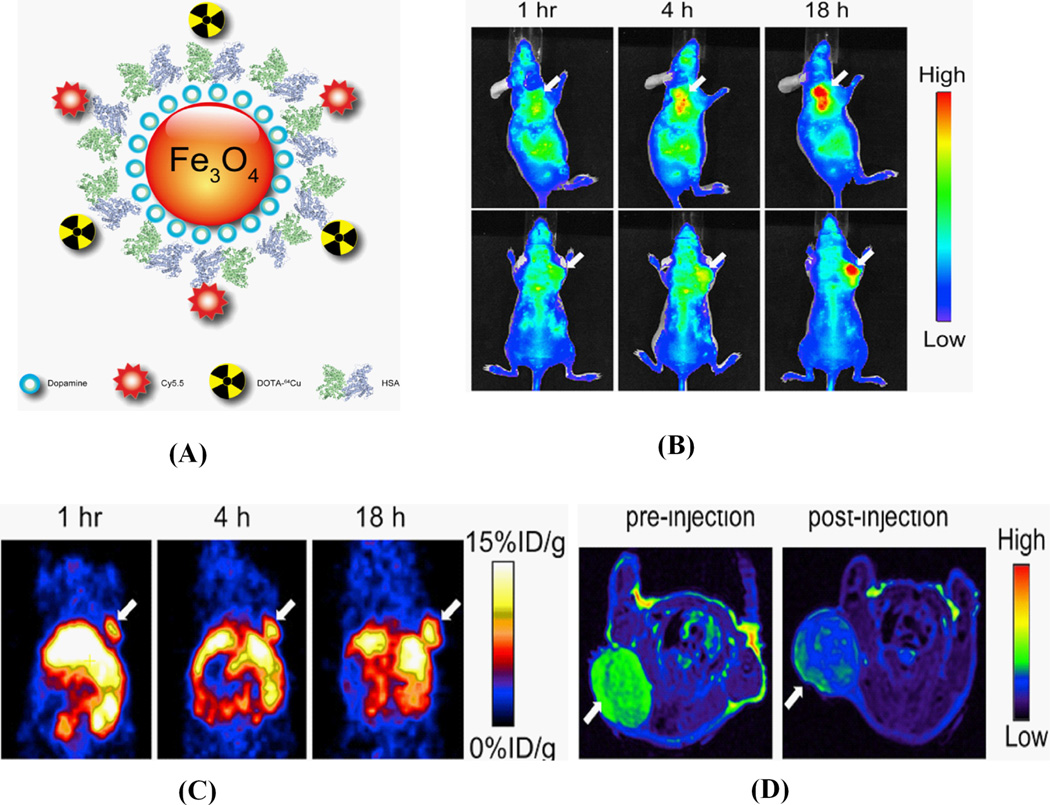
A multi-functional probe for PET/NIRF/MRI was recently reported by Xie et. al. based on dopamine-coated iron oxide nanoparticles. On the surface, human serum albumin (HAS) was encapsulated with dopamine and labeled with Cy5.5 and 64Cu-DOTA, and tested in a subcutaneous U87MG xenograft mouse model. In vivo PET/NIRF/MRI tri-modality imaging was performed, and ex vivo analysis with histological examinations was carefully conducted to investigate the in vivo behavior of the nanostructures. With the compact HAS coating, the HSA-IONPs demonstrated a prolonged circulation half-life, and showed massive accumulation in lesions, high extravasation rate, and low uptake of the particles by macrophages at the site of the tumor, as shown in Fig. 13 [124]
Figure 13.
(A) Schematic representation of triple functional probe HAS-IONPs for PET/Optical and MRI; (B) Representative in vivo NIRF images of mouse injected with probe. Images were acquired 1 h, 4 h and 18 h post injection; (C) in vivo PET imaging results of mouse after injection at 1 h, 4 h and 18 h and (D) MRI images acquired before and 18 h post injection. (Reprinted with the permission from 124).
Kimura et. al. reported the development of a dual-labeled probe conjugated with 64Cu-DOTA and Cy5.5 with a knottin peptide for the imaging of αvβ3 and αvβ5 integrin receptors. NIRF and PET imaging studies in tumor xenograft models showed that Cy5.5 conjugation significantly increased kidney uptake and retention compared to the knottin peptide labeled with 64Cu-DOTA alone. In the tumor, the dual-labeled 64Cu-DOTA/Cy5.5 knottin peptide showed decreased wash-out leading to significantly better retention (p < 0.05) compared to the 64Cu-DOTA-labeled knottin peptide [125].
More recently, Nam Taehwan et. al. developed tumor targeting chitosan nanoparticles for optical/MR imaging based on polymeric nanoparticle technology. Biocompatible and water-soluble glycol chitosan (MW 50 kDa) was chemically modified with 5-cholanic acid (CA), and conjugated with Cy5.5 and Gd3+-DOTA. When the targeted conjugate compound (Cy5.5-GC-Gd3+) was systemically administrated into the tail vein of tumor-bearing mice, large amounts of nanoparticles were successfully localized within the tumor, which was confirmed by non-invasive near-infrared fluorescence and MR imaging simultaneously. These results revealed that the dual-modality imaging probe has the potential to be used as an optical/MR dual imaging agent for cancer treatment [126].
This emerging field of multi-modality imaging using dual labeled probes promises to allow researchers to detect the same probe with multiple imaging techniques. The capability of cross-modality validation can provide more accurate and reliable data than with a single imaging modality alone. To date, technical and practical issues associated with dual-labeled probes and multi-modality imaging systems have made it more difficult for translation into clinical applications.
8. Summary and Future Prospects
Functional and molecular imaging has become increasingly more directed and specific, taking advantage of technical advances in various imaging modalities and novel molecular probes for every aspect of cancer. These powerful techniques have provided a meaningful approach to study and image the dynamics of biological processes at the molecular level. Continued progress our understanding of molecular biology has identified a number of cell surface receptors that can be used as molecular targets. Recent advances brought about by integrating diverse ideas in this inter-disciplinary field has generated novel constructs. In this review, we have presented numerous approaches for the design of exogenous probes that have been developed for their specific applications. Most of the probes have been successfully demonstrated in small animal models. Among these probes, a few have also reached the clinic. For future applications, we hope that the successes gained from pre-clinical imaging will facilitate their translation into the clinic to achieve improved diagnostic accuracy and monitoring of therapeutic response. The characterization of multiple targets, development of new probes, and advancements in imaging instruments promise to lead to more widespread applications. However, the single imaging modality may not be adequate to answer important scientific and clinical questions. Despite these challenges, we must keep in mind that inter-disciplinary research at the interface of imaging science, molecular biology, and probe chemistries can provide numerous uncharted opportunities and lead to the development of novel instruments and probes that will become valuable pre-clinical and clinical tools in the near future.
References
- 1.Weissleder R, Reimer P, Lee AS, Wittenberg J, Brady TJ. MR Receptor Imaging: Ultrasmall Iron Oxide Particles Targeted to Asialoglycoprotein Receptors. Am. J. Roentgenol. 1990;155:1161–1167. doi: 10.2214/ajr.155.6.2122660. [DOI] [PubMed] [Google Scholar]
- 2.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In Vivo Visualization of Gene Expression Using Magnetic Resonance Imaging. Nat. Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 3.Gambhir SS, Barrio JR, Phelps ME, Iyer M, Namavari M, Satyamurthy N, Wu L, Green LA, Bauer E, MacLaren DC, Nguyen K, Berk AJ, Cherry SR, Herschman HR. Imaging Adenoviral-directed Reporter Gene Expression in Living Animals with Positron Emission Tomography. Proc. Natl. Acad. Sci. 1999;96:2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayerhofer R, Araki K, Szalay AA. Monitoring of Spatial Expression of Firefly Luciferase in Transformed Zebrafish. J. Biolumin. Chemilumin. 1995;10:271–275. doi: 10.1002/bio.1170100503. [DOI] [PubMed] [Google Scholar]
- 5.Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD. Rapid and Quantitative Assessment of Cancer Treatment Response Using In Vivo Bioluminescence Imaging. Neoplasia. 2000;2:491–495. doi: 10.1038/sj.neo.7900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern J. Contrast-enhanced Ultrasound Imaging of Prostate Cancer. Rev. Urol. 2006;8:S29–S37. [PMC free article] [PubMed] [Google Scholar]
- 7.Contag CH, Bachmann MH. Advances in In Vivo Bioluminescence Imaging of Gene Expression. Annu. Rev. Biomed. Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 8.Soling A, Rainov NG. Bioluminescence Imaging In Vivo-application to Cancer Research. Expert Opin. Biol. Ther. 2003;3:1163–1172. doi: 10.1517/14712598.3.7.1163. [DOI] [PubMed] [Google Scholar]
- 9.Nahar M, Dutta T, Murugesan S, Asthana A, Mishra D, Rajkumar V, Tare M, Saraf S, Jain NK. Functional Polymeric Nanoparticles: an Efficient and Promising Tool for Active Delivery of Bioactives. Crit. Rev. Ther. Drug Carrier Syst. 2006;23:259–318. doi: 10.1615/critrevtherdrugcarriersyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Park K, Nam HY, Lee S, Kim K, Kwon KC. Polymers for Bioimaging. Prog. Polym. Sci. 2007;32:1031–1053. [Google Scholar]
- 11.Hama Y, Urano Y, Koyama Y, Kamiya M, Bernardo M, Paik RS, Shin IS, Paik CH, Choyke PL, Kobayashi H. A Target Cell-Specific Activatable Fluorescence Probe for In Vivo Molecular Imaging of Cancer Based on a Self-quenched Avidin-rhodamine Conjugate. Cancer Res. 2007;67:2791–2799. doi: 10.1158/0008-5472.CAN-06-3315. [DOI] [PubMed] [Google Scholar]
- 12.Bremer C, Tung CH, Bogdanov A, Jr., Weissleder R. Imaging of Differential Protease Expression in Breast Cancers for Detection of Aggressive Tumor Phenotypes. Radiology. 2002;222:814–818. doi: 10.1148/radiol.2223010812. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Wang J, Deng X, Sun H, Shi Z, Gu Z, Liu Y, Zhaoc Y. Biodistribution of Carbon Single-Wall Carbon Nanotubes in Mice. J. Nanosci. Nanotechnol. 2004;4:1019–1024. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue Biodistribution and Blood Clearance Rates of Intravenously Administered Carbon Nanotube Radiotracers. Proc. Natl. Acad. Sci. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa CH, McDevitt MR, Escorcia FE, Rey DA, Bergkvist M, Batt CA, Scheinberg DA. Synthesis and Biodistribution of Oligonucleotide-functionalized, Tumortargetable Carbon Nanotubes. Nano Lett. 2008;8:4221–4228. doi: 10.1021/nl801878d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benaron DA. The Future of Cancer Imaging. Cancer Metastasis Rev. 2002;21:45–78. doi: 10.1023/a:1020131208786. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem Rev. 2010 doi: 10.1021/cr900263j. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevick-Muraca EM, Houston JP, Gurfinkel M. Fluorescence-enhanced, Near Infrared Diagnostic Imaging with Contrast Agents. Curr. Opin. Chem. Biol. 2002;6:642–650. doi: 10.1016/s1367-5931(02)00356-3. [DOI] [PubMed] [Google Scholar]
- 19.Weissleder R, Mahmood U. Molecular Imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 20.Goetz M, Wang TD. Molecular Imaging in Gastrointestinal Endoscopy. 2010;138:828–833. doi: 10.1053/j.gastro.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Rosales RTM, Arstad E, Blower PJ. Nuclear Imaging of Molecular Processes in Cancer. Targ. Oncol. 2009;4:183–197. doi: 10.1007/s11523-009-0120-2. [DOI] [PubMed] [Google Scholar]
- 22.Wester HJ. Nuclear Imaging Probes: from Bench to Bedside. Clin. Cancer Res. 2007;13:3470–3481. doi: 10.1158/1078-0432.CCR-07-0264. [DOI] [PubMed] [Google Scholar]
- 23.Ross BD, Chenevert TL, Rehemtulla A. Magnetic Resonance Imaging in Cancer Research. Eur. J. Cancer. 2002;38:2147–2156. doi: 10.1016/s0959-8049(02)00387-8. [DOI] [PubMed] [Google Scholar]
- 24.Chan KW, Wong WT. Small Molecular Gadolinium (III) Complexes as MRI Contrast Agents for Diagnostic Imaging. Coord. Chem. Rev. 2007;251:2428–2451. [Google Scholar]
- 25.Lin Y, Weissleder R, Tung CH. Novel Near-Infrared Cyanine Fluorochromes: Synthesis, Properties, and Bioconjugation. Bioconjugate Chem. 2002;13:605–610. doi: 10.1021/bc0155723. [DOI] [PubMed] [Google Scholar]
- 26.Pierce MC, Javier DJ, Kortum RR. Optical Contrast Agents and Imaging Systems for Detection and Diagnosis of Cancer. Int. J. Cancer. 2008;123:1979–1990. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devous MD, Lowe JL, Sr., Payne JK. Dual-Isotope Brain SPECT Imaging with Technetium and Iodine-123: Validation by Phantom Studies. J. Nucl. Med. 1992;33:2030–2035. [PubMed] [Google Scholar]
- 28.Raymond KN, Pierre VC. Next Generation, High Relaxivity Gadolinium MRI Agents. Bioconjugate Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 29.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: Form and Function. Radiology. 2007;242:360–385. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 30.Bullok KE, Dyszlewski M, Prior JL, Pica CM, Sharma V, Piwnica-Worms D. Characterization of Novel Histidine-Tagged Tat-Peptide Complexes Dual-Labeled with (99m)Tc-Tricarbonyl and Fluorescein for Scintigraphy and Fluorescence Microscopy. Bioconjugate Chem. 2002;13:1226–1237. doi: 10.1021/bc025573a. [DOI] [PubMed] [Google Scholar]
- 31.Houston JP, Ke S, Wang W, Li C, Sevick-Muraca EM. Quality Analysis of In VivoNear-Infrared Fluorescence and Conventional Gamma Images Acquired Using a Dual-Labeled Tumor-Targeting Probe. J. Biomed. Opt. 2005;10 doi: 10.1117/1.2114748. 054010. [DOI] [PubMed] [Google Scholar]
- 32.Huber MM, Staubli AB, Kustedjo K, Gray MH, Shih J, Fraser SE, Jacobs RE, Meade TJ. Fluorescently Detectable Magnetic Resonance Imaging Agents. Bioconjugate Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 33.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A Multimodal Nanoparticle for Preoperative Magnetic Resonance Imaging and Intraoperative Optical Brain Tumor Delineation. Cancer Res. 2003;63:8122–8125. [PubMed] [Google Scholar]
- 34.Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Dendrimer-Based Nanoprobe for Dual Modality Magnetic Resonance and Fluorescence Imaging. Nano Lett. 2006;6:1459–1463. doi: 10.1021/nl060765q. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman RM. The Multiple Uses of Fluorescent Proteins to Visualize Cancer In Vivo. Nat. Rev. Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 36.Hama Y, Koyama Y, Urano Y, Choyke PL, Kobayashi H. Simultaneous Two-Color Spectral Fluorescence Lymphangiography with Near Infrared Quantum Dots to Map Two Lymphatic Flows from the Breast and the Upper Extremity. Breast Cancer Res. Treat. 2007;103:23–28. doi: 10.1007/s10549-006-9347-0. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H, Hama Y, Koyama Y, Barrett T, Regino CA, Urano Y, Choyke PL. Simultaneous Multicolor Imaging of Five Different Lymphatic Basins Using Quantum Dots. Nano Lett. 2007;7:1711–1716. doi: 10.1021/nl0707003. [DOI] [PubMed] [Google Scholar]
- 38.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. Fluorescence Confocal Endomicroscope for In VivoMicroscopy of the Upper and Lower Gastrointestinal Tract. Gastrointest. Endosc. 2005;62:686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Lam S, Standish B, Baldwin C, McWilliams A, leRiche J, Gazdar A, Vitkin AI, Yang V, Ikeda N, MacAulay C. In VivoOptical Coherence Tomography Imaging of Preinvasive Bronchial Lesions. Clin. Cancer Res. 2008;14:2006–2011. doi: 10.1158/1078-0432.CCR-07-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs RE, Cherry SR. Complementary Emerging Techniques: High-resolution PET and MRI. Curr. Opin. Neurobiol. 2001;11:621–629. doi: 10.1016/s0959-4388(00)00259-2. [DOI] [PubMed] [Google Scholar]
- 41.Te Velde EA, Veerman T, Subramaniam V, Ruers T. The Use of Fluorescent Dyes and Probes in Surgical Oncology. EJSO. 2010;36:6–15. doi: 10.1016/j.ejso.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M. Sentinel Node Mapping Guided by Indocyanine Green Fluorescence Imaging: a New Method for Sentinel Node Navigation Surgery in Gastrointestinal Cancer. Dig. Surg. 2008;25:103–108. doi: 10.1159/000121905. [DOI] [PubMed] [Google Scholar]
- 43.Klohs J, Wunder A, Licha K. Near-Infrared Fluorescent Probes for Imaging Vascular Pathophysiology. Basic Res. Cardiol. 2008;103:144–151. doi: 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- 44.Licha K, Olbrich C. Optical Imaging in Drug Discovery and Diagnostic Applications. Adv. Drug Delivery Rev. 2005;57:1087–1108. doi: 10.1016/j.addr.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable Imaging Probes with Amplified Fluorescent Signals. Chem. Commun. 2008;28:4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 46.Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. Semiconductor Quantum Dots and Metal Nanoparticles: Syntheses, Optical Properties, and Biological Applications. Anal. Bioanal. Chem. 2008;391:2469–2495. doi: 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- 47.Frangioni JV. In vivo Near-infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grotzinger C. Receptor-targeted Optical Imaging of Tumors with Near-infrared Fluorescent Ligands. Nat. Biotechnol. 2001;19:327–331. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 49.Kostenich G, Livnah N, Bonasera TA, Yechezkel T, Salitra Y, Litman P, Kimel S, Orenstein A. Targeting Small Cell Lung Cancer with Novel Fluorescent Analogs of Somatostatin. Lung Cancer. 2005;50:319–328. doi: 10.1016/j.lungcan.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Kostenich G, Oron-Herman M, Kimel S, Livnah N, Tsarfaty I, Orenstein A. Diagnostic Targeting of Colon Cancer Using a Novel Fluorescent Somatostatin Conjugate in a Mouse Xenograft Model. Int. J. Cancer. 2008;122:2044–2049. doi: 10.1002/ijc.23353. [DOI] [PubMed] [Google Scholar]
- 51.Ma L, Yu P, Veerendra B, Rold TL, Retzloff L, Prasanphanich A, Sieckman G, Hoffman TJ, Volkert WA, Smith CJ. In Vitro and In VivoEvaluation of Alexa Fluor 680-Bombesin-[7–14]NH2 Peptide Conjugate, a High-affinity Fluorescent Probe with High Selectivity for the Gastrin-releasing Peptide Receptor. Mol. Imaging. 2007;6:171–180. [PubMed] [Google Scholar]
- 52.Chen X, Conti PS, Moats R. A In vivo Near-infrared Fluorescence Imaging of Integrin αvβ3 in Brain Tumor Xenografts. Cancer Res. 2004;64:8009–8014. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- 53.Cheng Z, Wu Y, Xiong Z, Gambhir SS, Chen X. Near-infrared Fluorescent RGD Peptides for Optical Imaging of Integrin αvβ3 Expression in Living Mice. Bioconjugate Chem. 2005;16:1433–1441. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Cai W, Chen X. Near-infrared Fluorescence Imaging of Tumor Integrin αvβ3 Expression with Cy7-labeled RGD Multimers. Mol. Imaging Biol. 2006;8:226–236. doi: 10.1007/s11307-006-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly KA, Waterman P, Weissleder R. In vivo Imaging of Molecularly Targeted Phage. Neoplasia. 2006;8:1011–1018. doi: 10.1593/neo.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, Sage EH. Enhanced Expression of SPARC/Osteonectin in the Tumor-Associated Stroma of Non-small Cell Lung Cancer is Correlated with Markers of Hypoxia/acidity and with Poor Prognosis of Patients. Cancer Res. 2006;63:5376–5380. [PubMed] [Google Scholar]
- 57.Cybulsky MI, Gimbrone MA., Jr. Endothelial Expression of a Mononuclear Leukocyte Adhesion Molecule During Atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 58.Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Detection of Colonic Dysplasia In VivoUsing a Targeted Heptapeptide and Confocal Microendoscopy. Nat. Med. 2008;14:454–458. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Folli S, Westermann P, Braichotte D, Pelegrin A, Wagnieres G, van den Bergh H, Mach JP. Antibody-indocyanin Conjugates for Immunophotodetection of Human Squamous Cell Carcinoma in Nude Mice. Cancer Res. 1994;54:2643–2649. [PubMed] [Google Scholar]
- 60.Ballou B, Fisher GW, Waggoner AS, Farkas DL, Reiland JM, Jaffe R, Mujumdar RB, Mujumdar SR, Hakala TR. Tumor Labeling In VivoUsing Cyanine-conjugated Monoclonal Antibodies. Cancer Immunol. Immunother. 1995;41:257–263. doi: 10.1007/BF01517001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birchler M, Neri G, Tarli L, Halin C, Viti F, Neri D. Infrared Photodetection for the In Vivo Localisation of Phage-derived Antibodies Directed Against Angiogenic Markers. J. Immunol. Methods. 1999;231:239–248. doi: 10.1016/s0022-1759(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 62.Matter CM, Schuler PK, Alessi P, Meier P, Ricci R, Zhang D, Halin C, Castellani P, Zardi L, Hofer CK, Montani M, Neri D, Luscher TF. Molecular Imaging of Atherosclerotic Plaques Using a Human Antibody Against the Extra-domain B of Fibronectin. Circ. Res. 2004;95:1225–1233. doi: 10.1161/01.RES.0000150373.15149.ff. [DOI] [PubMed] [Google Scholar]
- 63.Birchler M, Viti F, Zardi L, Spiess B, Neri D. Selective Targeting and Photocoagulation of Ocular Angiogenesis Mediated by a Phage-Derived Human Antibody Fragment. Nat. Biotechnol. 1999;17:984–988. doi: 10.1038/13679. [DOI] [PubMed] [Google Scholar]
- 64.Ke S, Wen X, Gurfinkel M, Charnsangarvej C, Wallace S, Sevick-Muraca EM, Li C. Near-infrared Optical Imaging of Epidermal Growth Factor Receptor in Breast Cancer Xenografts. Cancer Res. 2003;63:7870–7875. [PubMed] [Google Scholar]
- 65.Citrin D, Scott T, Sproull M, Menard C, Tofilo PJ, Camphausen K. In Vivo Tumor Imaging Using a Near-infrared-labeled Endostatin Molecule. Int. J. Radiation Oncology Biol. Phys. 2004;58:536–541. doi: 10.1016/j.ijrobp.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 66.Licha K, Debus N, Ebert B, Vollmer-Emig S, Hasbach M, Sydow S, Stibenz D, Semmler W, Buhrer C, Schirner M, Tauber R. Optical Molecular Imaging of Lymph Nodes Using a Targeted Vascular Contrast Agent. J. Biomed. Optics. 2005;10 doi: 10.1117/1.2007967. 041205-1-041205-6. [DOI] [PubMed] [Google Scholar]
- 67.Bando T, Muguruma N, Ito S, Musashi Y, Inayama K, Kusaka Y, Tadatsu M, Ii K, Irimura T, Shibamura S, Takesako K. Basic Studies on a Labeled Anti-mucin Antibody Detectable by Infrared-fluorescence Endoscopy. J. Gastroenterol. 2002;37:260–269. doi: 10.1007/s005350200033. [DOI] [PubMed] [Google Scholar]
- 68.Goetz M, Ziebart A, Foersch S, Vieth M, Waldner MJ, Delaney P, Galle PR, Neurath MF, Kiesslich R. In vivo Molecular Imaging of Colorectal Cancer with Confocal Endomicroscopy of Epidermal Growth Factor Receptor. Gastroenterology. 2010;138:435–446. doi: 10.1053/j.gastro.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 69.Tung CH, Lin Y, Moon WK, Weissleder R. A Receptor-targeted Near-infrared Fluorescence Probe for In Vivo Tumor imaging. Chembiochem. 2002;3:784–786. doi: 10.1002/1439-7633(20020802)3:8<784::AID-CBIC784>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 70.Moon WK, Lin Y, O’Loughlin T, Tang Y, Kim DE, Weissleder R, Tung CH. Enhanced Tumor Detection Using a Folate Receptor-targeted Near-infrared Fluorochrome Conjugate. Bioconjugate Chem. 2003;14:539–545. doi: 10.1021/bc0340114. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Gryshuk A, Achilefu S, Ohulchansky T, Potter W, Zhong T, Morgan J, Chance B, Prasad PN, Henderson BW, Oseroff A, Pandey RK. A Novel Approach to a Bifunctional Photosensitizer for Tumor Imaging and Phototherapy. Bioconjugate Chem. 2005;16:1264–1274. doi: 10.1021/bc050177o. [DOI] [PubMed] [Google Scholar]
- 72.Stefflova K, Li H, Chen J, Zheng G. Peptide-Based Pharmacomodulation of a Cancer-Targeted Optical Imaging and Photodynamic Therapy Agent. Bioconjugate chem. 2007;18:379–388. doi: 10.1021/bc0602578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weissleder R, Tung CH, Mahmood U, Bognanov A. In Vivo Imaging of Tumors with Protease-activated Near-infrared Fluorescent Probes. Nat. Biotech. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 74.Ogawa M, Kosaka N, Longmire MR, Urano Y, Choyke PL, Kobayashi H. Fluorophore-Quencher Based Activatable Targeted Optical Probes for Detecting In Vivo Cancer Metastases. Mol. Pharmaceutics. 2009;6:386–395. doi: 10.1021/mp800115t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urano Y, Asanuma1 D, Hama Y, Koyama Y, Barrett T, Kamiya1 M, Nagano T, Watanabe T, Hasegawa A, Choyke PL, Kobayashi H. Selective Molecular Imaging of Viable Cancer Cells with pH-activatable Fluorescence Probes. Nat. Med. 2009;8:104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai W, Chen X. Nanoplatforms for Targeted Molecular Imaging in Living Subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 77.Cai W, Gao T, Hong H, Sun J. Applications of Gold Nanoparticles in Cancer Nanotechnology. Nanotechnol. Sci. Appl. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang YJ, Chang CH, Chang TJ, Yu CY, Chen LC, Jan ML, Luo TY, Lee TW, Ting G. Biodistribution, Pharmacokinetics and Micro SPECT/CT Imaging of 188Re-BMEDA-liposome in a C26 Murine Colon Carcinoma Solid Tumor Animal Model. Anticancer Res. 2007;27:2217–2225. [PubMed] [Google Scholar]
- 79.Chang CH, Stabin MG, Chang YJ, Chen LC, Chen MH, Chang TJ, Lee TW, Ting G. Comparative Dosimetric Evaluation of Nanotargeted 188Re-(DXR)-Liposome for Internal Radiotherapy. Cancer Biother. Radiopharm. 2008;23:749–758. doi: 10.1089/cbr.2008.0489. [DOI] [PubMed] [Google Scholar]
- 80.Hamoudeh M, Fessi H, Mehier H, Faraj AA, Canet-Soulas E. Dirhenium Decacarbonyl-loaded PLLA Nanoparticles: Influence of Neutron Irradiation and Preliminary In Vivo Administration by the TMT Technique. Int. J. Pharm. 2008;348:125–136. doi: 10.1016/j.ijpharm.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 81.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 82.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled Near-infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 83.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O’Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and Promise of FDG-PET Imaging for Cancer Patient Management and Oncologic Drug Development. Clin. Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 84.Higashi K, Ueda Y, Ayabe K, Sakurai A, Seki H, Nambu Y, Oguchi M, Shikata H, Taki S, Tonami H, Katsuda S, Yamamoto I. FDG PET in the Evaluation of the Aggressiveness of Pulmonary Adenocarcinoma: Correlation with Histopathological Features. Nucl. Med. Commun. 2000;21:707–714. doi: 10.1097/00006231-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 85.de Geus-Oei LF, Vander Heijden HF, Corstens FH, Oyen W. Predictive and Prognostic Value of FDG-PET in Non Small-cell Lung Cancer, Systematic Review. Cancer. 2007;110:1654–1664. doi: 10.1002/cncr.22979. [DOI] [PubMed] [Google Scholar]
- 86.Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, Lutolf UM, Steinert HC, Von Schulthess GK. Radiation Treatment Planning with an Integrated Positron Emission and Computer Tomography (PET/CT): a Feasibility Study. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:853–863. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 87.Leyton JV, Olafsen T, Lepin EJ, Hahm S, Bauer KB, Reiter RE, Wu AM. Humanized Radioiodinated Minibody for Imaging of Prostate Stem Cell Antigen-Expressing Tumors. Clin. Cancer Res. 2008;14:7488–7496. doi: 10.1158/1078-0432.CCR-07-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olafsen T, Cheung CW, Yazaki PJ, Li L, Sundaresan G, Gambhir SS, Sherman MA, Williams LE, Shively JE, Raubitschek AA, Wu AM. Covalent Disulfide-linked Anti-CEA Diabody Allows Site-specific Conjugation and Radiolabeling for Tumor Targeting Applications. Protein Eng. Des. Sel. 2004;17:21–27. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kobayashi H, Han ES, Kim IS, Le N, Rajagopal V, Kreitman RJ, Pastan I, Paik CH, Carrasquillo JA. Similarities in the Biodistribution of Iodine-labeled Anti-Tac Single-Chain Disulfide-Stabilized Fv Fragment and Anti-Tac Disulfide-Stabilized Fv Fragment-Comparison with its Single-chain analog. Nucl. Med. Biol. 1998;25:387–393. doi: 10.1016/s0969-8051(97)00228-x. [DOI] [PubMed] [Google Scholar]
- 90.Orlova A, Magnusson M, Eriksson TL, Nilsson M, Larsson B, Hoiden-Guthenberg I, Widstrom C, Carlsson J, Tolmachev V, Stahl S, Nilsson FY. Tumor Imaging Using a Picomolar Affinity HER2 Binding Affibody Molecule. Cancer Res. 2006;66:4339–4348. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 91.Wu AM. Antibodies and Antimatter: The Resurgence of Immuno-PET. J. Nucl. Med. 2009;50:2–5. doi: 10.2967/jnumed.108.056887. [DOI] [PubMed] [Google Scholar]
- 92.Cheng Z, de Jesus OP, Kramer DJ, De A, Webster JM, Gheysens O, Levi J, Namavari M, Wang S, Park JM, Zhang R, Liu H, Lee B, Syud FA, Gambhir SS. 64Cu-Labeled Affibody Molecules for Imaging of HER2 Expressing Tumors. Mol. Imaging Biol. 2009;25:1–9. doi: 10.1007/s11307-009-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reubi JC. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 94.McAfee JG, Neumann RD. Radiolabeled Peptides and Other Ligands for Receptors Overexpressed in Tumor Cells for Imaging Neoplasms. Nucl. Med. Biol. 1996;23:673–676. doi: 10.1016/0969-8051(96)00068-6. [DOI] [PubMed] [Google Scholar]
- 95.Reubi JC. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 96.Weiner RE, Thakur ML. Radiolabeled Peptides in the Diagnosis and Therapy of Oncological Diseases. Appl. Radiat. Isot. 2002;57:749–763. doi: 10.1016/s0969-8043(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 97.Okarvi SM. Peptide-based Radiopharmaceuticals: Future Tools for Diagnostic Imaging of Cancers and Other Diseases. Med. Res. Rev. 2004;24:357–397. doi: 10.1002/med.20002. [DOI] [PubMed] [Google Scholar]
- 98.Newton J, Deutscher SL. Phage Peptide Display. Handb. Exp. Pharmacol. 2008;185:145–163. doi: 10.1007/978-3-540-77496-9_7. [DOI] [PubMed] [Google Scholar]
- 99.Benedetti E, Morelli G, Accardo A, Mansi R, Tesauro D, Aloj L. Criteria for the Design and Biological Characterization of Radiolabeled Peptide-based Pharmaceuticals. Bio Drugs. 2004;18:279–295. doi: 10.2165/00063030-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 100.Aloj L, Morelli G. Design, Synthesis and Preclinical Evaluation of Radiolabeled Peptides for Diagnosis and Therapy. Curr. Pharm. Des. 2004;10:3009–3031. doi: 10.2174/1381612043383511. [DOI] [PubMed] [Google Scholar]
- 101.Chetanneau A, Gaucher L, Roullier A. 99mTechnetium-depreotide Thorax Uptake in 21 Patients with Pulmonary Nodule. Med. Nucl. 2003;27:265. [Google Scholar]
- 102.Menda Y, Kahn D. Somatostatin Receptor Imaging of Non Small Cell Lung Cancer with 99mTc Depreotide. Sem. Nucl. Med. 2002;32:92–96. doi: 10.1053/snuc.2002.31564. [DOI] [PubMed] [Google Scholar]
- 103.de Jong M, Valkema R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WAP, Bakker WH, Smith C, Pauwels S, Krenning EP. Somatostatin Receptor-targeted Radionuclide Therapy of Tumors: Preclinical and Clinical Findings. Semin. Nucl. Med. 2002;32:133–140. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 104.Kwekkeboom DJ, Krenning EP. Somatostatin Receptor Imaging. Semin. Nucl. Med. 2002;32:84–91. doi: 10.1053/snuc.2002.31022. [DOI] [PubMed] [Google Scholar]
- 105.Dijkgraaf I, Boerman OC, Oyen WJ, Corstens FH, Gotthardt M. Development and Application of Peptide-Based Radiopharmaceuticals. Anticancer Agents Med. Chem. 2007;7:543–551. doi: 10.2174/187152007781668733. [DOI] [PubMed] [Google Scholar]
- 106.Hong H, Zhang Y, Sun J, Cai W. Molecular Imaging and Therapy of Cancer with Radiolabeled Nanoparticles. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kehagias DT, Gouliamos AD, Smyrniotis V, Vlahos LJ. Diagnostic Efficacy and Safety of MRI of the Liver with Superparamagnetic Iron Oxide Particles (SHU555A) J. Magn. Reson. Imaging. 2001;14:595–601. doi: 10.1002/jmri.1224. [DOI] [PubMed] [Google Scholar]
- 108.Curtet C, Maton F, Havet T, Slinkin M, Mishra A, Chatal JF, Muller RN. Polylysine-Gd-DTPAn and Polylysine-Gd-DOTAn Coupled to Anti-CEA F(ab')2 Fragments as Potential Immunocontrast Agents: Relaxometry, Biodistribution, and Magnetic Resonance Imaging in Nude Mice Grafted With Human Colorectal Carcinoma. Invest. Radiol. 1998;33:752–761. doi: 10.1097/00004424-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of Tumor Angiogenesis In Vivoby αvβ3-targeted Magnetic Resonance Imaging. Nat. Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 110.Kresse M, Wagner S, Pfefferer D, Lawaczeck R, Elste V, Semmler W. Targeting of Ultrasmall Superparamagnetic Iron Oxide (USPIO) Particles to Tumor Cells In Vivo by Using Transferrin Receptor Pathways. Magn. Reson. Med. 1998;40:236–242. doi: 10.1002/mrm.1910400209. [DOI] [PubMed] [Google Scholar]
- 111.Torchilin VP. PEG-based Micelles as Carriers of Contrast Agents for Different Imaging Modality. Adv. Drug Deliv. Rev. 2002;54:235–252. doi: 10.1016/s0169-409x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 112.Zhang G, Zhang R, Wen X, Li L, Li C. Micelles Based on Biodegradable Poly(L-glutamic acid)-b-polylactide with Paramagnetic Gd Ions Chelated to the Shell Layer as a Potential Nanoscale MRI-visible Delivery. Biomacromolecules. 2008;9:36–42. doi: 10.1021/bm700713p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee HY, Jee HW, Seo SM, Kwak BK, Khang G, Cho SH. Diethylenetriaminepentaacetic Acid-Gadolinium (DTPA-Gd)-Conjugated Polysuccinimide Derivatives as Magnetic Resonance Imaging Contrast Agents. Bioconjug. Chem. 2006;17:700–706. doi: 10.1021/bc060014f. [DOI] [PubMed] [Google Scholar]
- 114.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI Ultrasensitive Drug Delivery Systems. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 115.Koch AM, Reynolds F, Kircher MF, Merkle HP, Weissleder R, Josephson L. Uptake and Metabolism of a Dual Fluorochrome Tat-nanoparticle in HeLa cells. Bioconjugate Chem. 2003;14:1115–1121. doi: 10.1021/bc034123v. [DOI] [PubMed] [Google Scholar]
- 116.Huh YM, Jun YW, Song HT, Kim S, Choi JS, Lee JH, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. In VivoMagnetic Resonance Detection of Cancer by Using Multifunctional Magnetic Nanocrystals. J. Am. Chem. Soc. 2005;127:12387–12391. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- 117.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang M. Optical and MRI Multifunctional Nanoprobe for Targeting Gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 118.Funovics M, Montet X, Reynolds F, Weissleder R, Josephson L. Nanoparticles for the Optical Imaging of Tumor E-selectin. Neoplasia. 2005;7:904–911. doi: 10.1593/neo.05352. [DOI] [PubMed] [Google Scholar]
- 119.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific Targeting of Nanoparticles by Multivalent Attachment of Small Molecules. Nat. Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 120.Quinti L, Weissleder R, Tung CH. A Fluorescent Nanosensor for Apoptotic Cells. Nano Lett. 2006;6:488–490. doi: 10.1021/nl0524694. [DOI] [PubMed] [Google Scholar]
- 121.Li G, Slansky A, Dobhal MP, Goswami LN, Graham A, Chen Y, Kanter P, Alberico RA, Spernyak J, Morgan J, Mazurchuk R, Oseroff A, Grossman Z, Pandey RK. Chlorophyll-a Analogues Conjugated with Aminobenzyl-DTPA as Potential Bifunctional Agents for Magnetic Resonance Imaging and Photodynamic Therapy. Bioconjugate Chem. 2005;16:32–42. doi: 10.1021/bc049807x. [DOI] [PubMed] [Google Scholar]
- 122.Ogawa M, Celeste AS, Regino CAS, Seidel J, Green MV, Xi W, Williams M, Kosaka N, Choyke PL, Kobayashi H. Dual-Modality Molecular Imaging Using Antibodies Labeled with Activatable Fluorescence and a Radionuclide for Specific and Quantitative Targeted Cancer Detection. Bioconjugate Chem. 2009;20:2177–2184. doi: 10.1021/bc900362k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu S, Jia B, Qiao R, Yang Z, Yu Z, Liu Z, Liu K, Shi J, Ouyang H, Wang F, Gao M. A Novel Type of Dual-modality Molecular Probe for MR and Nuclear Imaging of Tumor: Preparation, Characterization and In VivoApplication. Mol. Pharmaceutics. 2009;6:1074–1082. doi: 10.1021/mp900143a. [DOI] [PubMed] [Google Scholar]
- 124.Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. PET/NIRF/MRI Triple Functional Iron Oxide Nanoparticles. Biomaterials. 2010;11:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimura RH, Miao Z, Cheng Z, Gambhir SS, Cochran JR. A Dual-Labeled Knottin Peptide for PET and Near-Infrared Fluorescence Imaging of Integrin Expression in Living Subjects. Bioconjug.Chem. 2010;21:436–444. doi: 10.1021/bc9003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nam T, Park S, Lee SY, Park K, Choi K, Song IC, Han MH, Leary JJ, Yuk SA, Kwon IC, Kim K, Jeong SY. Tumor Targeting Chitosan Nanoparticles for Dual-Modality Optical/MR Cancer Imaging. Bioconjug. Chem. 2010 Mar 4; doi: 10.1021/bc900408z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]