Abstract
Microtubule-associated tumor suppressor gene (MTUS1, also known as mitochondrial tumor suppressor) is a recently identified tumor suppressor gene that has been implicated in several cancer types. The expression of MTUS1 gene leads to 5 known transcript variants and codes for 5 isoforms of Angiotensin II AT2 receptor interacting protein (ATIP). In this study, we first confirmed that the down-regulation of MTUS1/ATIP was a frequent event in oral tongue squamous cell carcinoma (OTSCC) and the premalignant lesion (leukoplakia). We further demonstrated that the down-regulation of MTUS1/ATIP was correlated with poor differentiation and enhanced proliferation (Ki67 proliferation index). Statistical analysis suggests that the down-regulation of MTUS1/ATIP was associated with reduced overall survival. Isoform specific quantitative RT-PCR assays revealed that ATIP1, ATIP3a and ATIP3b were the major isoforms of the MTUS1 gene products in oral tongue epithelial cells. Significant down-regulations were observed for all 3 ATIP isoforms in OTSCC as compared to matching normal tissues. In vitro functional study showed that the restoration of ATIP1 expression led to G1 arrest, apoptosis and reduction of cell proliferation in OTSCC cell lines. These ATIP1-induced cellular changes were accompanied by reduced phosphorylation of ERK1/2 and up-regulation of p53. Taken together, these data suggest that MTUS1 plays major roles in the progression of OTSCC, and may serve as a biomarker or therapeutic target for patients with OTSCC.
Keywords: ATIP, MTUS1, OTSCC, tumor suppressor, p53
1. Introduction
Oral tongue squamous cell carcinoma (OTSCC) is one of the most common sites for head and neck squamous cell carcinoma (HNSCC). An estimated 10,990 new cases of tongue cancer are expected each year, accounting for approximately 30% of all oral cavity and pharynx cancers (Jemal et al. 2010). OTSCC is significantly more aggressive than other forms of oral cancer, with a propensity for rapid local invasion and spread, and a high recurrence rate (Franceschi et al. 1993; Lydiatt et al. 1993; Yuen et al. 1999). OTSCC is characterized by genetic instabilities (Ye et al. 2007; Zhou et al. 2005; Zhou et al. 2004), including a frequent LOH at the chromosomal region 8p21.3-p22 (Ye et al. 2007). Microtubule-associated tumor suppressor gene (MTUS1, also known as mitochondrial tumor suppressor) is one of the candidate tumor suppressor genes that reside in this chromosomal region. It was initially identified as a potential tumor suppressor gene in pancreatic cancer (Seibold et al. 2003). The down-regulation of MTUS1 gene expression has also been documented in several cancer types (Bacolod and Barany 2010; Di Benedetto et al. 2006b; Louis et al. 2010; Rodrigues-Ferreira et al. 2009; Seibold et al. 2003; Zuern et al. 2010), including head and neck squamous cell carcinoma (HNSCC) (Ye et al. 2007). Our recent study suggested that the reduction of MTUS1 expression may be associated with advanced OTSCC (Zhou et al. 2006).
Alternative exon utilization of the MTUS1 gene leads to 5 known transcript variants that code for 5 different protein isoforms of Angiotensin II AT2 receptor interacting protein (ATIP1, ATIP2, ATIP3a, ATIP3b, ATIP4) (Di Benedetto et al. 2006a; Yu et al. 2009). Among these isoforms, ATIP1 and ATIP3a/b exhibit tumor suppressor function (Rodrigues-Ferreira et al. 2009; Seibold et al. 2003). ATIP1 is widely expressed in many different tissues (Di Benedetto et al. 2006a), and is transiently up-regulated during the initiation of cell differentiation and quiescence (Seibold et al. 2003). Our recent study demonstrated that ATIP1 expression was regulated by p53 at the transcriptional level (Chen et al. 2011). Functional analyses indicate that ATIP1 is an early component of the growth-inhibiting signaling cascade that interacts with the angiotensin II AT2 receptor. It inhibits the EGF-mediated ERK kinase activation and cell proliferation in an AT2-receptor dependent manner (Nouet et al. 2004; Seibold et al. 2003; Wruck et al. 2005). The initial evidence supporting the tumor suppressor function of ATIP3a/b comes from the study of the Xenopus Icis gene, a homolog of ATIP3a/b. Using inactivating antibodies, Ohi et al. found that absence of Icis caused excessive microtubule growth and inhibited spindle formation (Ohi et al. 2003), a function consistent with tumor suppressor activity. Frequent down-regulation of ATIP3a/b has recently been detected in a large cohort of breast cancer cases, and ATIP3a/b appeared to also regulate spindle dynamics and to promote prolonged mitosis in breast cancer cells (Rodrigues-Ferreira et al. 2009).
Although the tumor suppressor function of MTUS1/ATIP has been defined, its role in the initiation and progression of OTSCC is unclear. In the present study, we aim to assess the clinical significance of MTUS1/ATIP deregulation in patients with oral premalignancy lesion and OTSCC.
2. Material and Methods
2.1. Pooled-analysis to extract expression values of MTUS1/ATIP gene from existing microarray datasets
To conduct a pooled-analysis, microarray datasets for 33 OTSCC cases and 19 matching adjacent non-cancerous samples (Supplementary Table 1) were either generated from our previous study (Zhou et al. 2006) or downloaded from the GEO database (Ziober et al. 2006). The pooled-analysis was performed as described previously (Yu et al. 2008). In brief, the CEL files from all datasets were imported into the statistical software R 2.4.1 (R_Development_Core_Team 2006) using Bioconductor (Gentleman et al. 2004). The Robust Multi-Array Average (RMA) expression measures were computed for each microarray dataset after background correction and quantile normalization (Irizarry et al. 2003; Yu et al. 2008). Then, expression values of the overlapping probesets between U133A and U133 Plus 2.0 arrays were extracted. Probeset-level quantile normalization was performed across all samples to make the effect sizes similar between the datasets (Yu et al. 2008). The expression values for probesets corresponding to MTUS1/ATIP gene (212093_s_at, 212095_s_at, and 212096_s_at) were then extracted from each dataset. These probesets are not specific to the transcript variants of the MTUS1/ATIP gene, and they target the common regions of all the MTUS1/ATIP transcript variants. Relative mRNA level for MTUS1/ATIP were computed for each OTSCC sample as previously described (Liu et al. 2010).
2.2. Patients and tissues
The archived tissue samples from 80 cases of OTSCC, 27 cases of premalignant tongue (leukoplakia) and 13 normal tongue biopsies were utilized in this study (Supplementary Table 2). All OTSCC patients were diagnosed and received curative surgery between 1998 and 2006 at Department of Oral and Maxillofacial Surgery, the First Affiliated Hospital, Sun Yat-sen University. None of the patients received any form of adjuvant therapy prior to surgery. The tumor extent was classified according to the TMN system by UICC, and the tumor grade was classified according to the WHO classification of histological differentiation. Among 80 cases of OTSCC that we examined, follow-up results were available on 39 cases. Median duration of follow-up was 39 months (range 8–110 months). Survival was calculated based on the date of surgery and the date of latest follow-up (or death). This study was approved by the ethical committee of the First Affiliated Hospital, Sun Yat-Sen University.
2.3. Immunohistochemistry analysis
Immunohistochemistry was performed on 5 mm sections of formalin-fixed, paraffin-embedded tissue samples. Representative sections were first stained with H&E and histologically evaluated by a pathologist. Heat-mediated antigen retrieval was performed in EDTA buffer pH 9 in a water bath for 30 min. Immunohistochemical analysis was performed as described previously (Wang et al. 2009), using a commercially available kit (Invitrogen, Carlsbad, CA). In brief, endogenous peroxidase activity was quenched by incubation in a 9:1 methanol/30%hydrogen peroxide solution for 10 min at room temperature. Sections were then blocked with 10% normal serum for 10 min at 37 °C followed by incubation with an anti-MTUS1 antibody (Abnova) or an anti-Ki67 antibody (ABCam) at a dilution of 1:40 or 1:200 respectively, for 16 hr at room temperature. After washing three times in PBS, the sections were incubated with secondary antibody conjugated to biotin for 10 min at room temperature. After additional washing in PBS, the sections were incubated with streptavidin-conjugated horseradish peroxidase enzyme for 10 min at room temperature. After the final wash with PBS, antigen-antibody complexes were detected by incubation with a horseradish peroxidase substrate solution containing 3, 3'-diaminobenzidine tetrahydrochloride chromogen reagent, and counterstained with hematoxylin. Slides were rinsed in distilled water, cover-slipped using aqueous mounting medium, and allowed to dry at room temperature.
The relative intensities of the completed immunohistochemical reactions were evaluated by 3 independent trained observers who were unaware of the clinical data. All areas of tumor cells within each section were analyzed. Image-Pro Plus v6.0 (Media Cybernetics, USA) was used to score relative intensity. All tumor cells in ten random high power fields were measured for each case.
2.4. Cell culture, plasmid constructs and transfections
OTSCC cell lines (SCC-9, SCC-15, and UM1) were maintained in DMEM/F12 supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (GIBCO). Cells were cultured at 37°C in a humidified incubator containing 5% CO2. Expression vector containing the coding sequence of human ATIP1 was a gift from Dr. Stefan Seibold at University Medical Center, Medical Faculty University of Cologne (Seibold et al. 2003). For functional analysis, the ATIP1 expression vector or empty vector (pCDNA3, Invitrogen) was transfected into cells using Lipofectamine 2000 (Invitrogen).
2.5. Cell proliferation assays
Proliferation was measured using a MTT assay as described previously (Jiang et al. 2010). In brief, cells were seeded in quadruplicate in 96-well plates at the density of 5×103 cells per well. Cell proliferation was analyzed at 48 hr by incubation the cells with 1 mg/ml tetrazolium salt MTT (Sigma). Absorbance (A) at 570 nm was measured and cell inhibition rate was calculated as (1-Atreated/Acontrol) ×100%.
2.6. Flow cytometry-based apoptosis and cell cycle analysis
Cells were grown in 6-well plates to about 60% confluence and transiently transfected with the desired expression vector. For cell cycle analysis, the cells were harvested and resuspended in PBS and then fixed in ethanol at −20°C overnight. The cells were washed with PBS and resuspended in Staining Solution (50 μg/mL of propidium iodide, 1 mg/mL of RNase A, 0.1% Triton X-100 in PBS). The stained cells (1×105) were then analyzed with a flow cytometer (FACScan, Becton-Dickinson, CA, USA). The percentage of cells residing in the G0/G1 phase, S phase and G2/M phase were counted using ModiFit software and CellQuest programs. For apoptosis measurement, the cells were harvested and washed twice in PBS, and then resuspended in 500 μl of PBS plus AnnexinV-PE and 7-AAD (AnnexinV-PE/7-AAD staining kit, BioVision, Mountain View, CA). The stained cells (1×105) were then analyzed with a flow cytometer (FACScan, Becton-Dickinson, CA, USA).
2.7. Western Blot Analysis
Western blots were performed as described previously (Liu et al. 2009) using antibodies specific to MTUS1 (Abnova), ERK1/2, p-ERK1/2, p53 (Cell Signaling Technology, Beverly, MA), beta-actin (Sigma-Aldrich, USA), and a Immu-Star HRP Substrate Kit (BIO-RAD, USA). The intensities of the Western blot bands were quantified by image analyzing software Quantity One (Bio-Rad, USA).
2.8. ATIP isoform-specific quantitative RT-PCR
The expression of ATIP isoforms were determined in 6 pairs of frozen OTSCC and normal tongue tissue samples using ATIP isoform-specific quantitative RT-PCR assays described previously by Di Benedetto et. al. (Di Benedetto et al. 2006a). In brief, total RNA from tumor or normal samples was isolated using RNeasy Mini kit (Qiagen). First-strand cDNA was synthesized by MLV-RT (Promega) using random hexamer primers (Promega). ATIP isoform-specific quantitative RT-PCR was performed using exon-specific primer pairs corresponding to 5 ATIP isoforms (ATIP1, ATIP2, ATIP3a, ATIP3b, and ATIP4) (Di Benedetto et al. 2006a). All reactions were performed in triplicate. Melting curve analyses were performed to ensure the specificity of the quantitative RT-PCR reactions. The data analysis was performed using a modified 2−delta delta Ct method described by Di Benedetto (Di Benedetto et al. 2006a), with PPIA (peptidylprolyl isomerase A) as an internal reference.
2.9. Statistical analysis
Data were analyzed using the Statistical Package for the Social Science (SPSS, Chicago, IL), Version 17.0. Spearman Correlation Coefficient was used to assess correlations among the gene expression and clinical and histopathological parameters. One-way ANOVA and student's t-test was used to compare differences between groups. Kaplan-Meier plots were constructed to present the survival outcomes. Cox regression was used for both univariate and multivariate analysis. For all statistical analyses, p < 0.05 was considered statistically significant.
3. Results
3.1. The expression of MTUS1/ATIP in OTSCC and oral premalignancy
Pooled-analysis was performed on existing microarray datasets to determine the expression of MTUS1/ATIP at mRNA levels in OTSCC (n=33) and normal control samples (n=19). As illustrated in Supplementary Figure 1, MTUS1/ATIP is significantly down-regulated in OTSCC when compared to the normal control tissues (p = 0.0166).
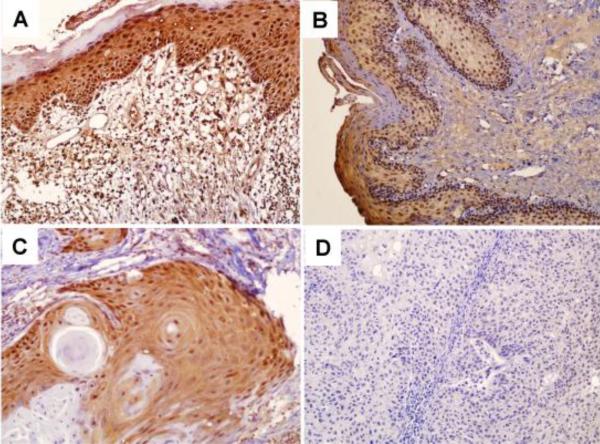
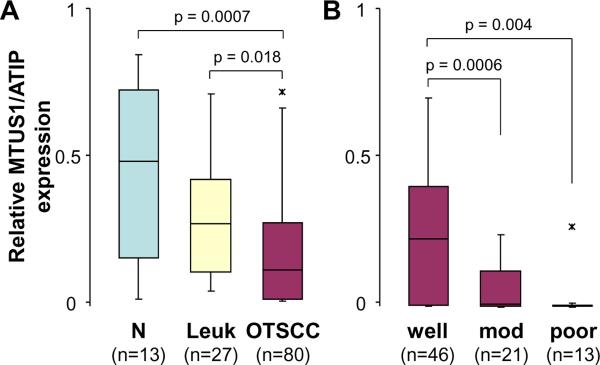
To confirm our observation and further elucidate the role of MTUS1/ATIP, the expression of the MTUS1/ATIP gene was examined by immunohistochemistry (IHC) in 80 cases of OTSCC, 27 cases of premalignant dysplasia (leukoplakia) and 13 normal tongue biopsies. As illustrated in Figure 1A, in normal tissues, MTUS1/ATIP was detectable in the entire epithelium (both cytoplasmic and nuclear staining) with the strongest staining observed in basal layers. As illustrated in Figure 2A, significant reductions in MTUS1/ATIP staining were observed in premalignant (Figure 1B) and cancer cells (Figure 1C and 1D). The loss of MTUS1/ATIP staining was observed in both cytoplasmic and/or nuclear compartments (Supplementary Figure 2). Among OTSCC cases, MTUS1/ATIP levels were significantly lower in poorly and moderately differentiated cases as compared to well differentiated OTSCC (Figure 2B). No difference in MTUS1/ATIP was observed in OTSCC cases of different pT, pN, and clinial stages (Supplementary Figure 3).
Figure 1.
Immunohistochemistry analyses of MTUS1/ATIP expression in normal tongue, premalignant dysplasia and OTSCC tissue samples. Immunohistochemistry analyses for MTUS1/ATIP were performed as described in Material and Methods on A: normal tongue mucosa (n = 13), B: premalignant dysplasia (leukoplakia, n = 27), C: well differentiated primary SCC (n = 46), and D: moderately to poorly differentiated primary SCC (n = 34). Representative Images (×200) were shown.
Figure 2.
Relative expression of MTUS1/ATIP in normal mucosa, premalignant dysplasia and OTSCC tissue samples. Box plots were presented for comparing the MTUS1/ATIP immunohistochemistry staining intensities in normal mucosa, premalignant dysplasia (leukoplakia) and OTSCC cases (A), and in OTSCC cases with different grade (differentiation) (B). The p-values were computed using one-way ANOVA. The boxes represent 25th to 75th percentile of the observations, and the lines in the middle of the box represent the median. The whiskers represent maximum (or minimum) observations below (or above) the 1.5 times of the interquartile range, respectively. Outliers are also indicated in the plots as *.
3.2. Correlation among MTUS1/ATIP expression and clinicopathological features in OTSCC
Correlations were tested among gene expression (e.g., MTUS1/ATIP and Ki67), clinical and pathological features in the OTSCC patient cohort (Table 1). As expected, strong correlations were observed among pT, pN, and the Clinical stage. Significant inverse correlations were observed between MTUS1/ATIP expression and Grade (differentiation) and Ki67 proliferation index. The Ki67 proliferation index is also correlated with Grade. Interestingly, the correlation was also observed between MTUS1/ATIP expression and gender. However, the biological significance of this observation is not clear.
Table 1.
Correlations among clinical and histopathological features of OTSCC†
| gender | age | grade | pT stage | pN stage | C stage | Ki67 | MTUS1/ATIP | |
|---|---|---|---|---|---|---|---|---|
| gender | 0.0932 | 0.2290* | 0.1220 | 0.2128 | 0.0808 | 0.0712 | 0.2274* | |
| age | 0.0211 | 0.0346 | 0.0051 | −0.0316 | 0.1801 | 0.0615 | ||
| grade | 0.0834 | 0.3144** | 0.1606 | 0.3106** | −0.3568** | |||
| pT stage | 0.5472** | 0.8297** | −0.0627 | 0.2059 | ||||
| pN stage | 0.8453** | 0.1285 | 0.1577 | |||||
| C stage | 0.0307 | 0.1155 | ||||||
| Ki67 | −0.2680* | |||||||
| MTUS1/ATIP |
Spearman Correlation Coefficients were presented.
pT: pathological T-stage; pN: pathological N-stage; C stage: clinical stage.
p < 0.05.
p < 0.01. The p values were computed using Fisher's transformed z-score test.
3.3. The prognostic value of MTUS1/ATIP deregulation for OTSCC patients
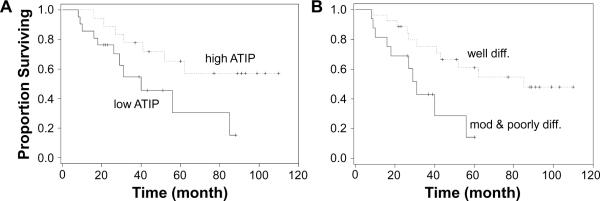
As illustrated in Figure 3A, a striking difference in prognosis was observed between the high MTUS1/ATIP expression group (mean survival = 61.8 months) and the low MTUS1/ATIP expression group (mean survival = 33 months). A statistically significant difference in survival was also observed when patients were grouped based on grade (differentiation) (Figure 3B). As illustrated in Supplementary Table 3>, univariate analysis indicated that grade and MTUS1/ATIP were significant prognostic factors for patients with OTSCC. These are consistent with the observed differences in overall survival. Multivariate analysis indicated that neither grade nor MTUS1/ATIP was independent prognostic factor. This may be due to the strong correlation between grade and MTUS1/ATIP. Alternatively, this may be due to our relative small sample size.
Figure 3.
The effects of MTUS1/ATIP expression on prognosis Kaplan-Meier plots of overall survival in patient groups defined by MTUS1/ATIP immunohistochemistry (A, High ATIP: higher than mean staining, Low ATIP: lower than mean staining) and differentiation (B). The differences in survival rates are statistically significant (p<0.05).
3.4. The relative down-regulation of the ATIP isoforms in OTSCC
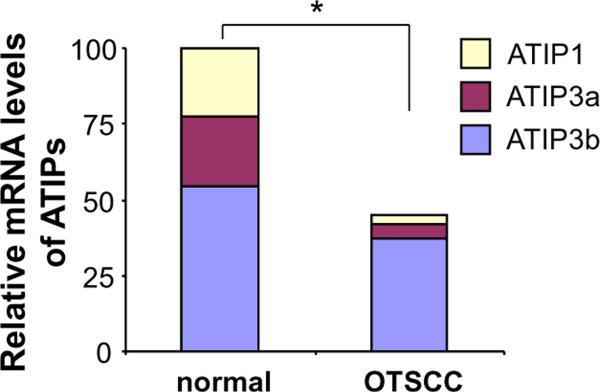
To assess the relative levels of each ATIP transcript variant in normal and OTSCC samples, ATIP isoform-specific quantitative RT-PCR were performed on 6 paired OTSCC and normal mucosa samples. As illustrated in Figure 4, a significant reduction of overall MTUS1/ATIP expression in OTSCC was confirmed. In normal mucosa, the relative proportions of ATIP1, ATIP3a, ATIP3b transcripts were 22.5%%, 22.6%, and 54.7%. In OTSCC, the relative proportions of these 3 transcripts were 14.1%, 27.8% and 56.8%. The ATIP2 and ATIP4 expression was minimal (<1% for both OTSCC and normal mucosa). Significant reductions in ATIP1, ATIP3a, ATIP3b were observed in OTSCC (86.5%, 77.5%, 32.3%, respectively, and p < 0.05), as compared with normal mucosa.
Figure 4. Relative expression of ATIP isoforms in OTSCC and adjacent normal tissues.
ATIP isoform-specific quantitative RT-PCR assays were performed as described on 6 paired OTSCC and adjacent normal mucosa samples. Relative proportions of ATIP1, ATIP3a, ATIP3b transcripts were presented for normal mucosa and OTSCC. Significant reductions in ATIP1, ATIP3a, ATIP3b and overall ATIP transcripts were observed in OTSCC as compared with normal mucosa. The ATIP2 and ATIP4 expression was minimal (less than 1% for both OTSCC and normal mucosa). * indicates p < 0.05.
3.5. The effects of MTUS1/ATIP on proliferation and apoptosis in OTSCC cell lines
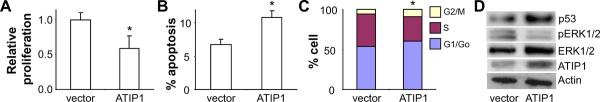
To validate the role of MTUS1/ATIP in OTSCC tumorigenesis, functional analyses were performed to test the effects of ATIP1 on proliferation, cell cycle and apoptosis. UM1 is one of the OTSCC cell lines that exhibit low MTUS1/ATIP expression (Supplementary Figure 4). As illustrated in Figure 5A, when UM1 cells were transfected with a ATIP1 expression vector, a statistically significant inhibition in cell proliferation was observed as compared to cells transfected with empty vector. As shown in Figure 5B, a statistically significant increase in apoptosis was observed in UM1 cells transfected with ATIP1 expression vector. Ectopic expression of ATIP1 in UM1 cells also led to changes in cell cycle, where statistically significant reduction in S and accumulation in G1/Go were observed in UM1 cells transfected with the ATIP1 expression vector (Figure 5C). Given that sub-G1/Go DNA content is indicative of apoptosis, these data also supported our observations on apoptosis. As shown in Figure 5D, while ectopic expression of ATIP1 increased the protein level of total ERK1/2, a clear decrease in phosphorylation of ERK1/2 (pERK1/2) was observed. This resulted in a statistically significant decrease in pERK/ERK ratio [1.00 ± 0.18 (ctrl) vs. 0.35 ± 0.26 (ATIP1 over-expression), p < 0.05, based on 3 independent experiments]. An enhanced expression of p53 was observed in UM1 cells transfected with the ATIP1 expression vector as compared to cells transfected with empty vector.
Figure 5.
The effects of ATIP1 on proliferation, cell cycle, apoptosis, levels of ERK1/2, pERK1/2 and p53 in OTSCC cell line UM1 cells were transiently transfected with an ATIP1 expression vector, or an empty vector. Cell proliferation (A), apoptosis (B), cell cycle (C) and the levels of ATIP1, ERK1/2, pERK1/2 and p53 (D) were measured. Data represents at least 3 independent experiments with similar results. * indicates p < 0.05.
4. Discussion
Our previous study identified one of the most frequent LOH (87.9%) in HNSCC located in a genomic region of ~ 7 Mb at 8p22-p21.3 (Ye et al. 2007). MTUS1/ATIP is one of the candidate tumor suppressor genes located in this region. Our preliminary analyses showed that 9 out of 10 HNSCC cell lines examined (include 5 OTSCC cell lines), and 7 out of 10 OTSCC tissue samples exhibited reduced expression of MTUS1/ATIP gene when compared to normal control. In the present study, we examined the expression of MTUS1/ATIP by a pooled-analysis of existing microarray datasets and a retrospective analysis of a large cohort of OTSCC and premalignancy patients. Our results demonstrate that down-regulation of MTUS1/ATIP is a frequent event during the progression of OTSCC. Further analyses reveal that down-regulation of MTUS1/ATIP correlates with poor differentiation and enhanced proliferation. These results suggested that deregulation of MTUS1/ATIP gene is involved in the loss of proliferative control and failure to undergo cellular differentiation during carcinogenesis. Furthermore, down-regulation of MTUS1/ATIP associates with short overall survival of the OTSCC patients. Thus, these findings underscore the critical contribution of MTUS1/ATIP deregulation in the tumorigenesis of OTSCC.
Alternative promoter utilization and alternative splicing are important features involved in the regulation of MTUS1/ATIP gene expression which lead to 5 different isoforms of protein products (ATIP1, ATIP2, ATIP3a, ATIP3b and ATIP4) (Di Benedetto et al. 2006a; Yu et al. 2009). Difference in tissue distribution has been reported previously for ATIP1, ATIP3a/b and ATIP4 (Di Benedetto et al. 2006a). While ATIP1 is ubiquitously expressed and ATIP4 is brain-specific, the relative levels of ATIP3a and ATIP3b vary among tissues. In saliva gland tissue, the level of ATIP3b is higher than ATIP3a. High levels of ATIP3a were observed in all other tissues previously examined as compared to ATIP3b (Di Benedetto et al. 2006a). Our results indicate that while ATIP2 and ATIP4 are not expressed to any significant extent, ATIP1, ATIP3a and ATIP3b are highly expressed in oral tongue mucosa cells. The ratio of ATIP3a/ATIP3b in oral mucosa cells was similar to that of the salivary gland, which may be due to the similar developmental lineages of cells from the salivary gland and oral mucosa. Compared to the normal mucosa, ATIP1, ATIP3a and ATIP3b were all significantly down-regulated in OTSCC. The most dramatic reduction was observed in ATIP1 (86.5%). The existence of ATIP2 is not entirely certain. As a result of alternative splicing, exon 3, which contains an in-frame stop codon, is incorporated into the ATIP2 transcript. This sequence feature makes ATIP2 transcript a candidate for nonsense-mediated mRNA decay (NMD). Indeed, it is absent (or weakly expressed) in all normal tissues examined (data not shown). Nevertheless, it is possible that this alternative splice to include exon 3 in the transcript may provide a mechanism to switch-off the expression of MTUS1/ATIP at posttranscriptional level.
While down-regulation of MTUS1/ATIP has been reported in OTSCC (and HNSCC), the effects of MTUS1/ATIP at the cellular level have not been documented before in OTSCC cells. Our in vitro study demonstrated that restoring ATIP1 expression in OTSCC cell lines induce G1/Go arrest, apoptosis and reduction in cell proliferation. This is consistent with our observed inverse correlation between MTUS1/ATIP expression and proliferation index (Ki67) in our OTSCC patient cohort. The knowledge of ATIP1 regulated molecular pathways is relatively limited. ATIP1 has previously been shown to be involved in the trans-inactivation of the EGF receptor and the subsequent inhibition of extracellular-regulated ERK kinase activity and cell proliferation (Nouet et al. 2004; Seibold et al. 2003; Wruck et al. 2005). We observed a reproducible up-regulation of ERK1/2 expression in UM1 cells upon the ATIP1 transfection. This is not consistent with previous observations in the prostate PC3 cell line, where ectopic expression of ATIP1 reduced total ERK1/2 levels (Louis et al. 2010). It is possible that this apparent difference may be due to differences in cancer types, or may be cell line specific. Nevertheless, a reduction in ERK1/2 phosphorylation was observed in our cell line upon the transfection of ATIP1. This is in agreement with the observation in PC3 cells (Louis et al. 2010) and the earlier observation in CHO cells (Nouet et al. 2004). Furthermore, the present study provided evidence suggesting that p53 may be an additional player that regulates the crosstalk between EGF signaling and the angiotensin II AT2-receptor signaling. We demonstrated that ectopic expression of ATIP1 led to up-regulation of p53. Interestingly, our recent study also demonstrated that ATIP1 expression is regulated by p53 at the transcriptional level (Chen et al. 2011). This evidence suggests a positive feedback loop of p53 and ATIP1 as well as a potential link between p53 and the signaling pathway(s) mediated by the angiotensin II AT2-receptor. More in-depth analysis will be needed to fully assess the functional relevance of these interactions and their contributions to tumorigenesis.
In summary, we described the expression pattern of MTUS1/ATIP in OTSCC. We demonstrated that the down-regulation of MTUS1/ATIP was associated with de-differentiation, enhanced proliferation and correlated with poor prognosis. Furthermore, the tumor suppressor function of ATIP1 is achieved, at least in part, by regulating the ERKs- and P53-dependent signaling path(s). Thus, our results provide evidence suggesting a critical role of MTUS1/ATIP in the tumorigenesis of OTSCC, and MTUS1/ATIP may serve as a biomarker or a novel therapeutic target for patients with OTSCC.
Supplementary Material
Highlights
-
1
Down-regulation of tumor suppressor MTUS1/ATIP is a frequent event in OTSCC.
-
1
Down-regulation of MTUS1/ATIP correlates with poor differentiation and enhanced proliferation, as well as reduced overall survival.
-
1
Restoration of MTUS1/ATIP expression led to G1 arrest, apoptosis and reduction of cell proliferation in OTSCC cell line.
Acknowledgements
This work was supported in part by grants from National Nature Science Foundation of China (NSFC81072228), Guangdong Natural Science Foundation (10151008901000093, S2011020002325), the International Cooperative Project of Science and Technology of Guangdong Province (1011420600001), and NIH PHS grant (CA139596). Y.J. is supported by a T32 training grant (T32DE018381). We thank Ms. Katherine Long for her editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacolod MD, Barany F. Gene dysregulations driven by somatic copy number aberrations-biological and clinical implications in colon tumors: a paper from the 2009 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2010;12:552–61. doi: 10.2353/jmoldx.2010.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Liu X, Wang C, Jin Y, Wang Y, Wang A, Zhou X. p53 regulates the expression of human angiotensin II AT2 receptor interacting protein (ATIP1) gene. Oncology Lett. 2011;2:919–22. doi: 10.3892/ol.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto M, Bieche I, Deshayes F, Vacher S, Nouet S, Collura V, Seitz I, Louis S, Pineau P, Amsellem-Ouazana D, Couraud PO, Strosberg AD, Stoppa-Lyonnet D, Lidereau R, Nahmias C. Structural organization and expression of human MTUS1, a candidate 8p22 tumor suppressor gene encoding a family of angiotensin II AT2 receptor-interacting proteins, ATIP. Gene. 2006a;380:127–36. doi: 10.1016/j.gene.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Di Benedetto M, Pineau P, Nouet S, Berhouet S, Seitz I, Louis S, Dejean A, Couraud PO, Strosberg AD, Stoppa-Lyonnet D, Nahmias C. Mutation analysis of the 8p22 candidate tumor suppressor gene ATIP/MTUS1 in hepatocellular carcinoma. Mol Cell Endocrinol. 2006b;252:207–15. doi: 10.1016/j.mce.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166:360–5. doi: 10.1016/s0002-9610(05)80333-2. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X. MicroRNA-7 targets insulin-like growth factor 1 receptor (IGF1R) in tongue squamous cell carcinoma cells. Biochem J. 2010 Sep 7; doi: 10.1042/BJ20100859. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang A, Lo Muzio L, Kolokythas A, Sheng S, Rubini C, Ye H, Shi F, Yu T, Crowe DL, Zhou X. Deregulation of manganese superoxide dismutase (SOD2) expression and lymph node metastasis in tongue squamous cell carcinoma. BMC Cancer. 2010;10:365. doi: 10.1186/1471-2407-10-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yu J, Jiang L, Wang A, Shi F, Ye H, Zhou X. MicroRNA-222 Regulates Cell Invasion by Targeting Matrix Metalloproteinase 1 (MMP1) and Manganese Superoxide Dismutase 2 (SOD2) in Tongue Squamous Cell Carcinoma Cell Lines. Cancer Genomics Proteomics. 2009;6:131–9. [PMC free article] [PubMed] [Google Scholar]
- Louis SN, Chow L, Rezmann L, Krezel MA, Catt KJ, Tikellis C, Frauman AG, Louis WJ. Expression and function of ATIP/MTUS1 in human prostate cancer cell lines. Prostate. 2010;70:1563–74. doi: 10.1002/pros.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiatt DD, Robbins KT, Byers RM, Wolf PF. Treatment of stage I and II oral tongue cancer. Head Neck. 1993;15:308–12. doi: 10.1002/hed.2880150407. [DOI] [PubMed] [Google Scholar]
- Nouet S, Amzallag N, Li JM, Louis S, Seitz I, Cui TX, Alleaume AM, Di Benedetto M, Boden C, Masson M, Strosberg AD, Horiuchi M, Couraud PO, Nahmias C. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279:28989–97. doi: 10.1074/jbc.M403880200. [DOI] [PubMed] [Google Scholar]
- Ohi R, Coughlin ML, Lane WS, Mitchison TJ. An inner centromere protein that stimulates the microtubule depolymerizing activity of a KinI kinesin. Dev Cell. 2003;5:309–21. doi: 10.1016/s1534-5807(03)00229-6. [DOI] [PubMed] [Google Scholar]
- R_Development_Core_Team . R Foundation for Statistical Computing. 2006. R: A language and environment for statistical computing. [Google Scholar]
- Rodrigues-Ferreira S, Di Tommaso A, Dimitrov A, Cazaubon S, Gruel N, Colasson H, Nicolas A, Chaverot N, Molinie V, Reyal F, Sigal-Zafrani B, Terris B, Delattre O, Radvanyi F, Perez F, Vincent-Salomon A, Nahmias C. 8p22 MTUS1 gene product ATIP3 is a novel anti-mitotic protein underexpressed in invasive breast carcinoma of poor prognosis. PLoS One. 2009;4:e7239. doi: 10.1371/journal.pone.0007239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold S, Rudroff C, Weber M, Galle J, Wanner C, Marx M. Identification of a new tumor suppressor gene located at chromosome 8p21.3-22. Faseb J. 2003;17:1180–2. doi: 10.1096/fj.02-0934fje. [DOI] [PubMed] [Google Scholar]
- Wang A, Liu X, Sheng S, Ye H, Peng T, Shi F, Crowe DL, Zhou X. Dysregulation of heat shock protein 27 expression in oral tongue squamous cell carcinoma. BMC Cancer. 2009;9:167. doi: 10.1186/1471-2407-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wruck CJ, Funke-Kaiser H, Pufe T, Kusserow H, Menk M, Schefe JH, Kruse ML, Stoll M, Unger T. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64. doi: 10.1161/01.ATV.0000150662.51436.14. [DOI] [PubMed] [Google Scholar]
- Ye H, Pungpravat N, Huang BL, Muzio LL, Mariggiò MA, Chen Z, Wong DT, Zhou X. Genomic assessments of the frequent LOH region on 8p22-p21.3 in head and neck squamous cell carcinoma. Cancer Genet Cytogenet. 2007;176:100–6. doi: 10.1016/j.cancergencyto.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Liu X, Ye H, Zhou X. Genomic characterization of the human mitochondrial tumor suppressor gene 1 (MTUS1): 5' cloning and preliminary analysis of the multiple gene promoters. BMC Res Notes. 2009;2:109. doi: 10.1186/1756-0500-2-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Ye H, Chen Z, Ziober BL, Zhou X. Dimension reduction and mixed-effects model for microarray meta-analysis of cancer. Front Biosci. 2008;13:2714–20. doi: 10.2741/2878. [DOI] [PubMed] [Google Scholar]
- Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK, Ho CM. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg. 1999;177:90–2. doi: 10.1016/s0002-9610(98)00294-3. [DOI] [PubMed] [Google Scholar]
- Zhou X, Jordan RCK, Li Y, Huang BL, Wong DT. Frequent allelic imbalance at 8p and 11q22 in oral cavity and oropharyngeal epithelial dysplastic lesions. Cancer Genet Cytogen. 2005;161:86–9. doi: 10.1016/j.cancergencyto.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Zhou X, Mok SC, Chen Z, Li Y, Wong DT. Concurrent analysis of loss of heterozygosity (LOH) and copy number abnormality (CNA) for oral premalignancy progression using the Affymetrix 10K SNP mapping array. Hum Genet. 2004;115:327–330. doi: 10.1007/s00439-004-1163-1. [DOI] [PubMed] [Google Scholar]
- Zhou X, Temam S, Oh M, Pungpravat N, Huang BL, Mao L, Wong DT. Global expression-based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia. 2006;8:925–32. doi: 10.1593/neo.06430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober AF, Patel KR, Alawi F, Gimotty P, Weber RS, Feldman MM, Chalian AA, Weinstein GS, Hunt J, Ziober BL. Identification of a gene signature for rapid screening of oral squamous cell carcinoma. Clin Cancer Res. 2006;12:5960–71. doi: 10.1158/1078-0432.CCR-06-0535. [DOI] [PubMed] [Google Scholar]
- Zuern C, Heimrich J, Kaufmann R, Richter KK, Settmacher U, Wanner C, Galle J, Seibold S. Down-regulation of MTUS1 in human colon tumors. Oncol Rep. 2010;23:183–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.