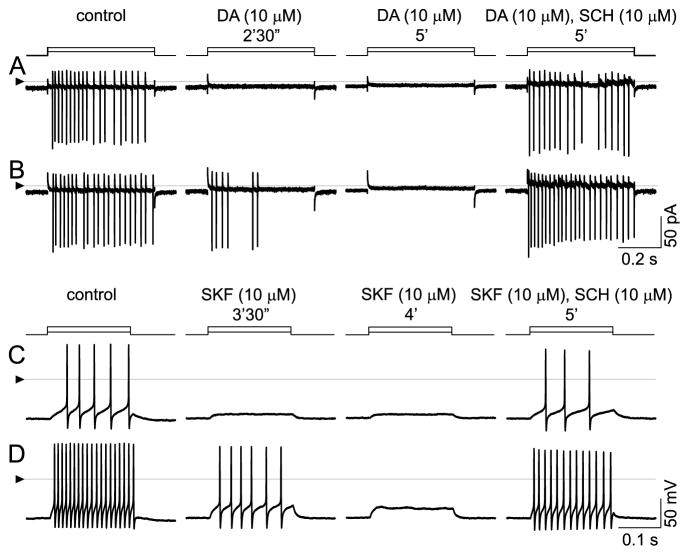
Figure 4.
Inhibition by dopamine and by SKF-38393, and reversal of these effects by SCH-23390. A, B: Cell-attached, voltage-clamp mode at 33 °C; currents appear as vertical lines due to slow time base, with downward deflections occurring at peak depolarization of each spike. Spikes elicited by stepwise changes in patch electrode voltage (5 mV in A, 10 mV in B). Solution continuously superfused over cell by U-tube microperfusion. Spikes in control solution (left, “control”) are blocked by inclusion of 10 μM dopamine (middle). Loss of spikes is complete within 2.5 min after onset of dopamine application at lower stimulus step size, but only partial at the higher step size. Spikes are completely blocked by 5 min after dopamine first reached the recording bath. Addition of SCH-23390 (so that the superfusate contains 10 μM dopamine and 10 μM SCH) blocks the response to dopamine at both stimulus strengths. C, D: Inhibition by SKF-38393. Ruptured-patch, current-clamp mode at 34 °C; 200-msec injections of constant current (10 pA in C, and 30 pA in D). Solution continuously superfused over cell by U-tube microperfusion. As in cell-attached recordings, spike firing is continuous during both stimulus pulses in control solution (left, “control”) and is inhibited during same stimulus pulses by 10 μM SKF-38393 (middle, “SKF 10 μM”). Spikes are first lost at low stimulus strength and subsequently lost at higher stimulus strength, too. SCH-23390 (i.e., perfusate containing 10 μM SKF and 10 μM SCH) blocks the response to SKF. Triangles at left show reference level for all traces in each row (zero-current in A, B; zero-voltage in C, D).