Abstract
Vertebrates can detect light intensity changes in vastly different photic environments, in part, because post-receptoral neurons undergo “network adaptation”. Previous data implicated dopaminergic, cAMP-dependent inhibition of retinal ganglion cells in this process, yet left unclear how this occurs, and whether this occurs in darkness versus light. To test for light- and dopamine-dependent changes in ganglion cell cAMP levels in situ, we immunostained dark- and light-adapted retinas with anti-cAMP antisera, in the presence and absence of various dopamine receptor ligands. To test for direct effects of dopamine receptor ligands and membrane-permeable protein kinase ligands on ganglion cell excitability, we recorded spikes from isolated ganglion cells in perforated-patch whole-cell mode, before and during application of these agents by microperfusion. Our immunostainings show that light, endogenous dopamine, and exogenous dopamine elevate ganglion cell cAMP levels in situ by activating D1-type dopamine receptors. Our spike recordings show that D1-type agonists and 8-bromo cAMP reduce spike frequency and curtail sustained spike firing, and that these effects entail protein kinase A activation. These effects resemble those of background light on ganglion cell responses to light flashes. Network adaptation could thus be produced, to some extent, by dopaminergic modulation of ganglion cell spike generation, a mechanism distinct from modulation of transmitter release onto ganglion cells or of transmitter-gated currents in ganglion cells. Combining these observations, with results obtained in studies of photoreceptor, bipolar, and horizontal cells, indicates that all three layers of neurons in the retina are equipped with mechanisms for adaptation to ambient light.
Keywords: Retina, light adaptation, network adaptation, contrast sensitivity, filtering, dopamine, protein kinase A
One of several remarkable ways in which vertebrate retinas contribute to vision is to adjust their dynamic range to ambient stimuli. A well-known illustration of this is the decline in light sensitivity as mean light levels increase. This adjustment is functionally important because it allows luminance contrast to be detected over a wide range of background light intensities, and because it abates response saturation (Shapley and Enroth-Cugell, 1984). In studies performed over the past 40 years, this adjustment has been found to be complex in at least two respects. First, it is achieved at moderate and bright background intensities by events in more than one population of cells, namely, light adaptation in photoreceptors (Baylor and Hodgkin, 1974) and network adaptation in interneurons and retinal ganglion cells (Barlow and Levick, 1969; Sakmann and Filion, 1972; Green et al., 1975). Second, network adaptation can occur at lower light intensities than does photoreceptor light adaptation (Pirenne, 1958; Sakmann and Filion, 1972; Enroth-Cugell and Shapley, 1973; Green et al., 1975; Ashmore and Falk, 1980), yet it can remain tonically effective, even at brighter intensities, for long periods of time (e.g., hours of daylight).
Modulation of ganglion cell light sensitivity by light that does not adapt photoreceptors, and by brighter light, might result from changes in the release of a neuromodulatory transmitter. Numerous observations indicate that dopamine is this type of transmitter. For example, light stimulates intraretinal dopamine release (Kramer, 1971), exogenous dopamine inhibits ganglion cells in situ (Straschill and Perwein, 1969; Glickman et al., 1982), and depletion or destruction of dopamine-releasing interneurons augments reflex responses to brightness (Häggendahl and Malmfors, 1965; Lin and Yazulla, 1994). Thus, several types of observations have together raised the possibility that light reduces ganglion cell spike firing via dopamine receptor activation.
To date, one study has shown that dopamine can inhibit spiking in dissociated retinal ganglion cells (Liu and Lasater, 1994). This study indicated that dopamine responses entail an elevation of cyclic 3′, 5′ adenosine monophosphate (cAMP), and concluded that inhibition results from reducing voltage-gated Ca2+ current, without affecting voltage-gated Na+ or K+ currents. These results were unexpected because previous investigators found that retinal ganglion cell cAMP levels were not changed by dopamine (Young and Dowling, 1989) or by adapting lights (Orr et al., 1976). Moreover, while dopamine activates cAMP-dependent protein kinase (PKA) in various neurons, this kinase does not necessarily produce inhibition by reducing voltage-gated Ca2+ currents (e.g., Schiffmann et al., 1995). Below, we re-examine whether light and dopamine augment cAMP levels in retinal ganglion cells. We also test whether dopamine receptor ligands inhibit ganglion cells under conditions that block voltage-gated Ca2+ currents, and whether spike inhibition by dopamine entails PKA activation. Some of these results have appeared in an abstract (Vaquero and Ishida, 2000).
METHODS
Species
Goldfish (Carassius auratus; 9–16 cm in body length) were used for this study, because several studies have suggested that their ganglion cells should respond to changes in dopamine release. In particular, dopaminergic neurons extend vesicle-containing processes into the ganglion cell and optic fiber layers (Yazulla and Zucker, 1988), dopamine receptors have been localized in the inner plexiform layer and on ganglion cell somata (Mora-Ferrer et al., 1999), and dopamine release is Ca2+-dependent (Sarthy and Lam, 1979). Fish were obtained from a commercial fish farm (Dutchman Creek, Merced CA) and maintained outdoors, in a 300-gallon holding tank, without artificial lighting. Fish were sacrificed by cervical/spinal transection and pithed. Eyes were rapidly excised, and retinas isolated, as described below. All animal care and experimental protocols conformed to guidelines of the Animal Use and Care Administrative Advisory Committee of the University of California, Davis.
Light- and dark-adaptation
A circadian oscillator can modulate retinal dopamine release (Dubocovich, 1983). Therefore, at least two weeks prior to experiments, fish were transferred to indoor holding tanks, so that their exposure to light could be controlled. For nearly all of the experiments reported here, room lights (50 μW/cm2) were turned on at 7 AM, and off at 7 PM. “Light-adapted” retinas were then dissected under room light at 10 AM (i.e., after a 3-hr exposure to room light during actual day). For comparison, some “dark-adapted” retinas were collected at 10 PM (i.e., after 3 hrs in darkness at actual night). Other dark-adapted retinas were collected from fish that were maintained for 3 hrs in a completely darkened tank, after having been transferred there at 10 AM.
For some experiments, the room lights in the indoor-tank facility were turned on at 1 AM, and off at 1 PM, everyday for a minimum of 2 weeks. Dark-adapted retinas were then collected at 4 PM (i.e., after 3 hrs in complete darkness at subjective night). For comparison, light-adapted retinas were collected from fish that were transferred to room light at 4 PM, and allowed to swim freely for 3 hrs. Results obtained at subjective night were indistinguishable from those obtained at actual night; results obtained during subjective day were indistinguishable from those obtained during actual day. We therefore refer to retinas herein as either dark- or light-adapted, without specifying actual versus subjective time.
After isolation, retinas were either dissociated into suspensions of cells for patch-clamp recordings, or processed for immunostaining using the procedures described below. Isolation, dissociation, incubation in pharmacological agents, and aldehyde fixation of dark-adapted retinas were performed entirely under infra-red illumination, with the aid of infra-red image-converting goggles. Light-adapted retinas were isolated and processed under room lighting.
Immunostaining
Aldehyde fixation
Freshly isolated retinas were either immersed in aldehyde fixative, or aldehyde-fixed after incubation in solutions that contained dopamine receptor ligands (see Results). The fixative contained 4% paraformaldehyde, 5% sucrose (w/v) and 150 μM CaCl2, in phosphate buffered saline (PBS, pH 7.4; #10010-031, GIBCO, Grand Island, NY). Retinas were fixed for 90 min at room temperature, and then rinsed three times (10-min each) in PBS containing 5% sucrose and 150 μM CaCl2. These retinas were either immunostained and viewed in whole-mount, or cryosectioned, immunostained, and viewed as transretinal sections.
Frozen sections
Retinas were cryoprotected by immersion in 30% sucrose for 1–12 hrs, cut into pieces no larger than 5 mm across, transferred to mounting medium [one part Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) plus two parts of 20% sucrose in PBS], frozen in liquid nitrogen, and sectioned on a cryostat at 8–10 μm thickness. Sections were mounted on Superfrost/Plus slides (Fisher Scientific; Santa Clara, CA), and stored at −20 °C. The immunostaining protocol was started within a few hours thereafter.
Immunostaining
Sections and whole-mounts were rinsed six times (5-min each) in PBS, and then incubated for 1 hour in a blocking solution that consisted of PBS supplemented with 10% normal goat serum and 0.5% Triton X-100. These sections and whole-mounts were incubated overnight at 4 °C, in (a) an affinity-purified rabbit antiserum directed against cyclic 3′, 5′ adenosine monophosphate (#116820; Calbiochem-Novabiochem; San Diego, CA); (b) a control (cAMP-preabsorbed) antiserum made by storing a mixture of 200 nM cAMP and the anti-cAMP antiserum for 1 hour at room temperature (Steiner et al., 1972); or (c) a second control antiserum made by storing a mixture of 200 nM cGMP and the anti-cAMP antiserum for 1 hour at room temperature. After ten 5-min rinses in PBS, sections and whole-mounts were incubated in a Cy3-conjugated anti-rabbit IgG (#111-165-003; Jackson ImmunoResearch Laboratories; West Grove, PA) for 1 hour in darkness. Lastly, sections and whole-mounts were rinsed in PBS, covered by FluorSave mounting medium (Calbiochem-Novabiochem) and glass coverslips, and imaged on a laser scanning confocal microscope (Model #TCS-SP, Leica; Deerfield, IL). Primary and secondary antisera were diluted immediately before use, to final concentrations of 1:300–1:1000 and 1:300, respectively, in PBS containing 5% normal goat serum and 0.1% Triton X-100. Test (anti-cAMP) and control (pre-incubated) antisera were applied at identical dilutions to sections cut from the same retinas. Pairs of control and test sections (e.g., dark- versus light-adapted, dopamine receptor agonist versus mixture of agonist plus antagonist, etc.) were imaged with identical photomultiplier gains and pinhole diameters. A Z-line slice depth of 580 nm was routinely used. The Adobe Photoshop (v.5.5) and Illustrator (v.9.0) software systems (Adobe Systems Inc., San Jose CA) were used to montage digital micrographs.
Curent-Clamp, Voltage-Clamp, and Data Analysis
Spikes (viz., action potentials) and voltage-gated Ca2+ currents were recorded with a patch-clamp amplifier (Axopatch 1D, Axon Instruments, Union City, CA) in current- and voltage-clamp mode, respectively. Recordings were made from ganglion cells that were dissociated and identified as described previously (Ishida and Cohen, 1988). For reasons stated in the Results, cells were dissociated without visible light, under infrared illumination only, from fish kept in darkness for a 3-hr period that commenced with the beginning of the dark phase of their dark:light cycle. Recordings were made within 12 hrs after these dissociations.
All recordings were made in perforated-patch mode (Horn and Marty, 1988). To distinguish between perforated- and ruptured-patch mode, Lucifer Yellow CH di-potassium (Aldrich, Milwaukee, WI) was routinely included at 0.2 mg/mL in the recording electrode solution. After formation of cell-attached mode, formation of perforated patch mode was signalled by a decrease in access resistance and increase in capacitive current transient amplitude, with no observable Lucifer fill. Subsequently applying a brief pulse of suction to the interior of the electrode holder resulted in the formation of ruptured patch mode, signalled by a further decrease in access resistance, an increase in capacitive current transient amplitude, and a brilliant Lucifer fill. Therefore, after recordings were completed in the present study, cells were examined for dye exclusion under epifluorescence illumination. Cells found then to be Lucifer-filled were not used for any of the measurements reported here.
Patch electrodes were pulled from borosilicate glass capillaries to tip resistances of 5 MΩ. For spike recordings, the tips of these pipettes were filled with “pipette solution” that contained (in mM): 15 NaCl, 123 KOH, 17 KCl, 0.25 CaCl2, 2.6 MgCl2, 1.5 BAPTA, and 5 HEPES. In some recordings, the concentrations of NaCl and CaCl2 in the pipette solution were 10 and 1 mM, respectively; this made no qualitative difference in the results. The pH was adjusted to 7.4 with HCl, and the osmolality was adjusted with sucrose to 260 mOsmol/kg. The pipette shanks were filled with this solution after addition at 1:1000 of a solution containing 2 mg Amphotericin B (Sigma; St Louis, MO) plus 3 mg Pluronic F-127 (Molecular Probes; Eugene, OR) in 60 μL DMSO (Sigma).
The control “bath solution” contained (in mM): 110 NaCl, 3.5 KCl, 2.5 CaCl2, 1 mM MgCl2, 10 D-glucose, and 5 HEPES. The pH was adjusted to 7.4 with NaOH, and the osmolality was adjusted with sucrose to 280 mOsmol/kg.
Voltage-gated Ca2+ currents were recorded at membrane potentials similar to those traversed during spikes. For this purpose, currents were activated by depolarizations from a holding potential of −62 mV to test potentials between −40 and 0 mV. The pipette solution contained (in mM): 120 CsOH, 15 NaCH3SO3, 0.34 CaCl2, 2.6 MgCl2, 1 EGTA, 5 HEPES. The pH was adjusted to 7.4 with methanesulfonic acid, the osmolarity was adjusted with sucrose to 284 mOsm, and the patch-perforating agent was amphotericin B (included as described above). The bath solution containined (in mM): 120 NaCl, 3 CsCl, 30 tetraethylammonium Cl, 2.5 CaCl2, 1 MgCl2, 10 D-glucose, 5 HEPES. The pH was adjusted to 7.4 with NaOH, and the osmolality was adjusted with sucrose to 300 mOsm. Voltage-gated Ca2+ currents were blocked (in current- and voltage-clamp experiments) by decreasing the bath Ca2+ to 0.1 (or 0.2) mM, and increasing the Mg2+ concentration to 2.4 (or 3.4) mM (see Results). In these recordings, voltage-gated Na+ current was blocked by 1 μM tetrodotoxin, and voltage-gated K+ currents were blocked by use of K+-free pipette and bath solutions, and by inclusion of 30 mM tetraethylammonium in the bath (Hidaka and Ishida, 1998).
Experiments were performed at room temperature (ca. 23 °C). Current stimulus generation, voltage-jump protocols, data acquisition, and some offline data analysis were performed with the pCLAMP system (versions 6.0.3 and 8.1.01, Axon Instruments, Union City, CA). Spikes were elicited by constant current pulses, and voltage-gated Ca2+ currents were activated by step-wise depolarizations. To minimize the possibility of current-clamp response variability among different cells due to differences in resting potential, small holding currents were used to set resting potentials to around −70 mV at the beginning of recordings. Spikes were collected in control and test solutions without changing these holding currents. Times that elapsed between spikes in individual spike trains were measured with the Mini Analysis Program (v.4.3.2, Synaptosoft Inc.; Leonia, NJ). In both current- and voltage-clamp experiments, the patch-clamp amplifier output was analog-filtered by the 4-pole Bessel filter of the amplifier (fc = 2 kHz), and digitally sampled at 2–5 kHz. The recording chamber was grounded via an agar bridge, and all membrane potentials were corrected for liquid junction potentials due to differences between the bath and pipette solution compositions.
Reagents
Test agents were dissolved in bath solution on the day of each experiment. To minimize oxidation, dopamine was dissolved in bath solution that was supplemented with 1 mM ascorbic acid and 0.1 mM EDTA. All other test solutions were prepared by dissolving agents into bath solution without ascorbate or EDTA. Test solutions were applied to cells by U-tube microperfusion (Krishtal and Pidoplichko, 1980), and to guard against mechanical artifacts, control spikes were recorded during U-tube microperfusion of the solution that each test agent was dissolved in.
Test agents were obtained from the following sources: dopamine, BAPTA, and ascorbic acid (Sigma); CaCl2 (BDH; Dorset, England); SCH-23390, SKF-38393, and sulpiride (Research Biochemicals International; Natick, MA); cAMP, cGMP, and 8-bromo-cAMP (Calbiochem-Novabiochem); Rp-5,6-DCl-cBIMPS (5,6-Dichloro-1-β-D-ribofuranosylbenz-imidazole-3′, 5′-cyclic monophosphorothioate, Rp-isomer; Biolog Life Science Institute; Bremen, Germany). These compounds were either dissolved in bath or pipette solution, at their final concentration, or they were diluted in these solutions from stock solutions made in water (or ethanol, in the case of sulpiride) at 300–1000 times the final concentration.
RESULTS
Two sets of data are presented here. The first addresses whether light or dark increases retinal ganglion cell cAMP levels in situ, and whether dopamine mediates this effect. The second shows effects of dopamine receptor activation on ganglion cell excitability.
Light elevates cAMP levels in retinal ganglion cells
Dark- and light-adapted retinas were immunostained with affinity-purified polyclonal antisera directed against cAMP. Dark-adapted retinas were collected from goldfish that were kept in complete darkness for 3 hours during actual night, subjective night, or actual morning (see Methods). Light-adapted retinas were collected from fish that were allowed to swim freely under room light for 3 hours during actual morning, actual night, or subjective night (see Methods). Shorter light-adaptation times were not used because our patch-clamp recordings showed that D1 receptor agonists elicit responses in ganglion cells within 1–2 min (see below), and retinas could not be aldehyde fixed rapidly enough to resolve cAMP changes within such short times. Cells were examined in the pericentral retina, midway between the optic disk and retinal perimeter. Each of the immunostaining results described below was confirmed in retinas isolated from a minimum of three fish, unless noted otherwise.
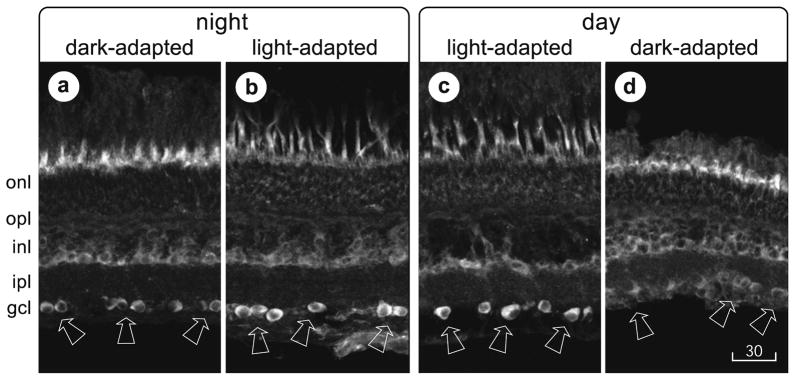
In dark-adapted retinas, cAMP-like immunoreactivity was found in the inner segments of photoreceptors that were cone-like in size and shape (Figs, 1a, 1d, 2a, 4d). This agreed with previous reports (Farber et al., 1981), and no attempt was made to determine if subtypes of cones were selectively stained. In the same retinas, cAMP-like immunoreactivity was either absent in retinal ganglion cells (Figs, 1a, 1d, 2b, 4d), or present in a few ganglion cells (result not illustrated).
Fig 1.
cMP in light-adapted retinal ganglion cells. Transretinal cryosections were immunostained with anti-cAMP antiserum (1:300 in a, b; 1:1000 in c, d) and imaged on a laser scanning confocal microscope. Dark-adapted retinas were isolated and fixed under infrared illumination 3 hr after sunset (a) or from fish transferred to complete darkness for a 3 hr period that began 3 hr after sunrise (d). Light-adapted retinas were isolated and fixed under room light 3 hr after sunrise (c) or from fish exposed to room light for a 3 hr period that began 3 hr after sunset (b). The intensity of fluorescence that passed through a 590 nm long-pass filter was displayed in gray scale in this figure and in Figures 3 and 4, with the brightest fluorescence appearing white. cAMP-like immunoreactivity is most vivid at the level of cone photoreceptor inner segments in dark-adapted retinas and in ganglion cell layer in light-adapted retinas. cAMP-like immunoreactivity in photoreceptors is reduced in light-adapted retinas. Arrows point to some of the ganglion cell somata in each section. Magnification is identical in all panels. onl, Outer nuclear layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer. Scale bar, 30 μm.
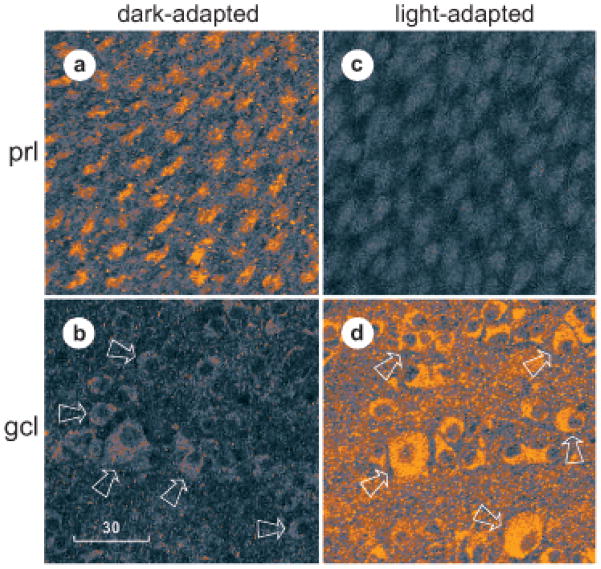
Fig 2.
Optical sections through retina of a fully dark-adapted fish (a, b) that was dissected under infrared illumination at 3 hr after sunset and a lightadapted fish (c, d) that was dissected under room light at 3 hr after sunrise. Each retina was fixed and immunostained as in Figure 1, then viewed in flat-mount at different transretinal levels at fixed lateral coordinates. Plane of section is at level of cone inner segments in photoreceptor layer (prl) in a and c and at level of ganglion cell somata (gcl) in b and d. The intensity of fluorescence that passed through a 590 nm long-pass filter was transformed into CMYK color space in this figure, with the dimmest fluorescence appearing light blue and the brightest fluorescence appearing orange. All sections (a–d) were imaged at identical settings of photomultiplier gain and pinhole diameter. Arrows point to some of the ganglion cell somata in b and d. A curved row of somata can be seen in b, surrounded by the four arrows at the left. Two rows of somata can be seen in d, one between the top pair of arrows and the other between the middle pair of arrows. Magnification is identical in all panels. Scale bar, 30 μm.
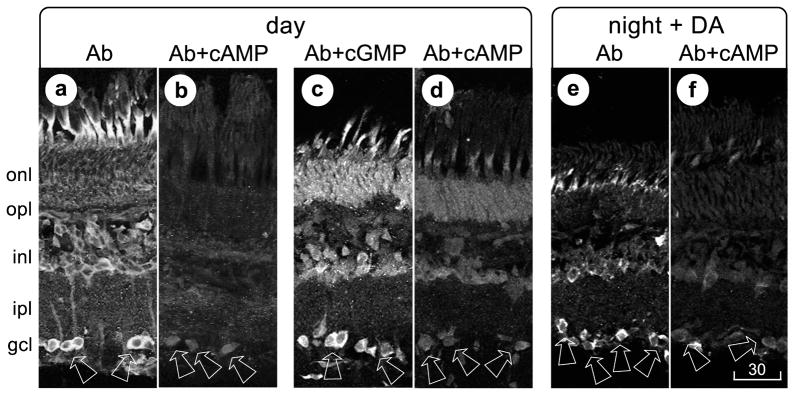
Fig 4.
Controls, using retinas that were sectioned, processed, and displayed as in Figure 1. a, b, One pair of sections was cut from a light-adapted retina, then incubated with anti-cAMP antiserum (a) or with anti-cAMP antiserum that was preincubated in cAMP (b). c, d, Another pair of sections was cut from another light-adapted retina, then incubated with anti-cAMP antiserum that was preincubated in cGMP (c) or with anti-cAMP antiserum that was preincubated in cAMP (d). e, f, A third retina was dark-adapted and incubated for 30 min in darkness in 30 μM dopamine. Sections cut from this retina were incubated with anti-cAMP antiserum (e) or with anti-cAMP antiserum that was preincubated in cAMP (f). Magnification is identical in all panels. Scale bar, 30 μm.
In light-adapted retinas, nearly all of the somata in the ganglion cell layer were stained (Figs, 1b, 1c, 2d, 3a, 4a). The stained somata were indistinguishable in diameter (10–25 μm) and shape (round to gourd-shaped) from those filled by retrograde transport of markers injected into the optic nerve (Ishida and Cohen, 1988), and from those immunostained by antibodies directed against a voltage-gated Na+-channel epitope (Yoshikawa et al., 2000). These somata also resembled the ganglion cell somata in which dopamine elevates cAMP (see below). The somata in the ganglion cell layer that did not exhibit cAMP-like immunoreactivity in light-adapted retinas were small in diameter (< 10 μm), like cells that do not exhibit voltage-gated Na+-channel-like immunoreactivity (Yoshikawa et al., 2000), and like displaced amacrine cell somata (Marc et al., 1990). Where the latter, blood vessels, and axon fascicles were absent, rows of immunostained somata could be seen (Figs, 1b, 1c, 2d, 3a, 4a). Serial optical sections through flat-mounted retinas showed that adjacent somata in the ganglion cell layer were immunostained without a noticeable bias for size or spatial distribution (e.g., Fig. 2c). These flat mounts, like the vertical sections in Fig. 1, also showed that cone inner segments were brightly stained in dark-adapted retinas but not in light-adapted retinas (compare Figs. 2a and 2c), whereas ganglion cells were brightly stained in light-adapted retinas but not in dark-adapted retinas (compare Figs. 2b and 2d).
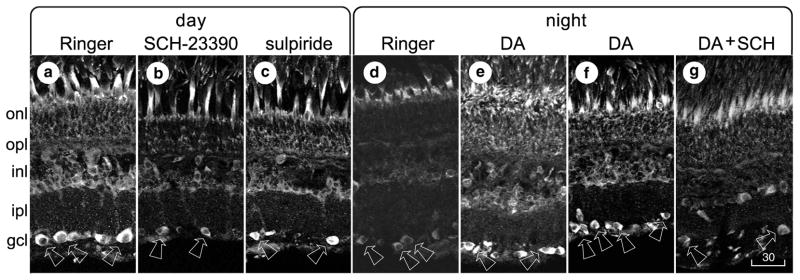
Fig 3.
Receptor profile, using retinas that were dark- or light-adapted, then sectioned, processed, and displayed as in Figure 1. a–c, Pieces of a single light-adapted retina were incubated for 20 min in normal Ringer’s solution (a), 10 μM SCH-23390 (b), or 10 μM sulpiride (c). d, e, Pieces of a single dark-adapted retina were incubated for 30 min in either normal Ringer’s solution (d) or 30 μM dopamine (e). f, g, Pieces of another dark-adapted retina were incubated for 30 min in 30 μM dopamine (f) or for 10 min in 30 μM dopamine, followed by 20 min in 30 μM dopamine plus 10 μM SCH-23390 (g). Magnification is identical in all panels. Scale bar, 30 μm.
The immunostaining of photoreceptors and ganglion cells appeared to be cAMP-specific for the following reasons. First, identical staining patterns were obtained with another anti-cAMP antiserum (#20-CR60, Fitzgerald Industries, Concord MA; results not illustrated). Second, no cells were stained by the anti-cAMP antiserum after it was pre-incubated with an excess of cAMP (see Methods; Figs. 3b, 3d, 3f). Third, exogenous cGMP did not alter the staining pattern (Fig. 3c).
Endogenous and exogenous dopamine elevates cAMP levels in retinal ganglion cells
Several investigators have reported that light triggers dopamine release from the retina (e.g., Kramer, 1971), and that dopamine stimulates retinal cAMP production (e.g., Brown and Makman, 1972). Recently, D1-type dopamine receptors have been localized to fish retinal ganglion cell somata (Wagner and Behrens, 1993; Mora-Ferrer et al., 1999). We therefore tested the possibility that light can elevate ganglion cell cAMP levels by stimulating endogenous dopamine release.
First, light-adapted retinas were cut into pieces and incubated either in control bath solution or in bath solution that contained dopamine receptor antagonists. Incubation of light-adapted retinas in a D1-type receptor antagonist (SCH-23390, 10 μM) abolished cAMP-like immunoreactivity in ganglion cells (compare Figs. 4a and 4b). A D2-type receptor antagonist (sulpiride, 10 μM) did not produce this effect (n=1; compare Figs. 4a and 4c).
Second, dark-adapted retinas were cut into pieces and incubated either in control bath solution or in bath solution that contained dopamine, for 30 min (see Dearry and Burnside, 1989; Hampson et al., 1992). Divalent cations that are typically used to block synaptic transmission were not added to the incubation media, because certain divalents (notably Co2+) have been found to increase retinal neuron responses to dopamine (Cohen, 1982), and because ganglion cell responses to dopamine receptor agonists were studied separately in the absence of synaptic inputs (see below). cAMP-like immunoreactivity was observed in ganglion cells in dark-adapted retinas incubated in 30 μM dopamine (Figs. 3e, 4e, 4f). This immunoreactivity resembled that observed in light-adapted retinas (Figs. 1b, 1c, 2d), in terms of the cells that were stained. As in light-adapted retinas that were not treated with dopamine, ganglion cells in dark-adapted, dopamine-treated retinas were not stained by antiserum that was pre-incubated in cAMP (Fig. 3f). Moreover, no cAMP-like immunoreactivity was observed in ganglion cells in pieces of dark-adapted retinas that were incubated for 10 min in 30 μM dopamine, and then incubated for 20 min in a mixture of 30 μM dopamine and 10 μM SCH-23390 (compare Figs. 4f and 4g). This effect is consistent with the block by SCH-23390 of light-induced increases in cAMP immunoreactivity, and with effects of SCH-23390 on intracellularly recorded dopamine responses (see below).
In some retinas that were exposed to dopamine, cAMP-like immunoreactivity was observed at the level of cone inner segments (Figs. 3e, 4f). This may reflect activation of D1 receptors that have been detected in fish photoreceptors by binding of SCH-23390 (Wagner and Behrens, 1993), in situ hybridization (Mora-Ferrer et al., 1996), and immunostaining (Mora-Ferrer et al., 1999).
Despite electrophysiological evidence that light elevates cAMP levels in cone-driven horizontal cells (e.g., Piccolino et al., 1984), we never observed cAMP-like immunoreactivity in horizontal cells in our preparations, either in dark-adapted retinas that were incubated in dopamine (Figs. 3, 4) or in light-adapted retinas (Figs. 1, 3, 4). For that matter, we are unaware of any previous immunostaining evidence that light increases cAMP levels in horizontal cells. We have no explanation for this apparent discrepancy. Light-sensitivity and synapse viability were evident in our preparations because light reduced photoreceptor cAMP levels and increased ganglion cell cAMP levels (Figs. 1, 2). We tested the possibility that an avid phosphodiesterase or muted adenylyl cyclase keeps horizontal cell cAMP levels below those in cones and ganglion cells (by incubating retinas in mixtures of dopamine, 10 μM forskolin, and either 2 mM isobutylmethylxanthine or 10 mM theophylline; cf. Dowling and Watling, 1981), and still observed no cAMP-like immunoreactivity in horizontal cells. Lastly, we did not observe cAMP-like immunoreactivity in horizontal cells in retinas fixed with glutaraldehyde rather than formaldehyde (results not illustrated).
D1-type receptor activation reduces retinal ganglion cell excitability
Having found that illumination elevates cAMP levels in retinal ganglion cells, and that this response is blocked by a D1-type receptor antagonist, we next tested whether D1-type dopamine receptor activation, and elevation of cytoplasmic cAMP, affect retinal ganglion cell excitability. These recordings incorporated the following considerations. First, to preclude effects of pharmacological agents on presynaptic cells (Hedden and Dowling, 1978; Shiells and Falk, 1985; Yamada and Saito, 1988; Heidelberger and Matthews, 1994; Hare and Owen, 1995), and to preclude the possibility that dopamine effects on homologous or heterologous coupling of ganglion cells (Vaney, 1991) could contribute to the results reported here, we measured the effect of D1-type receptor activation on responses to current injections into isolated ganglion cells (see Methods). Second, to perform these recordings under conditions identical to those examined by immunostaining above, and thus to minimize variability in spiking and dopamine responses that might arise from light-induced release of endogenous dopamine (or other modulators), all cells were dissociated under infrared illumination, from fish that were kept in complete darkness for 3 hours after the beginning of actual or subjective night (see Methods and Fig. 1). Third, to preserve second messenger cascades and protein kinase activities, all recordings were performed in perforated-patch mode (see Methods). Finally, because SCH-23390 blocked both light- and dopamine-stimulated cAMP increases in ganglion cells, spikes were recorded before and during application of the D1-type receptor agonist, SKF-38393, and in some cases, dopamine itself.
SKF-38393 (1–30 μM) and dopamine (0.3–3 μM) abolished spikes elicited by small amounts of exogenous current (e.g., compare recordings at left of Figs. 5a, b), and reduced the number of spikes elicited by intermediate current intensities (e.g., middle and right of Figs. 5a, b). These effects could have been characterized with brief depolarizations if the decrease in spike number resulted only from a decrease in spike frequency. However, cells that spiked continuously during prolonged current injections in control solution failed to spike toward the end of the same current injections during agonist applications (Figs. 5–8), and the “recruitment time” (i.e., the time-to-peak) of the first spike in each train increased (Figs. 5d, 6d, 7d, 8e). Because control recruitment times as long as approximately 100 msec were observed during small current injections, current stimulus durations of 150–500 msec were used to examine spike timing in both control and test solutions. Because only one or two spikes were usually elicited by small and intermediate stimuli in test solutions (Figs. 5–8), spike frequency was calculated from the inverse of the time that elapsed between the first two spikes elicited by each depolarization (Figs. 5–8). Control spike frequencies were calculated in the same way, particularly because control spikes accommodated during the long depolarizing steps used here (Figs. 5a, 6a, 7a, 8a). To guard against variability due to jitter, cells were depolarized several times at each stimulus intensity in all solutions, and mean values of the control and test spike latencies and frequencies were compared (Figs. 5–8). Lastly, cells were not depolarized more often than once per 4 sec, to allow voltage-gated Na+ current to recover from inactivation (Hidaka and Ishida, 1998).
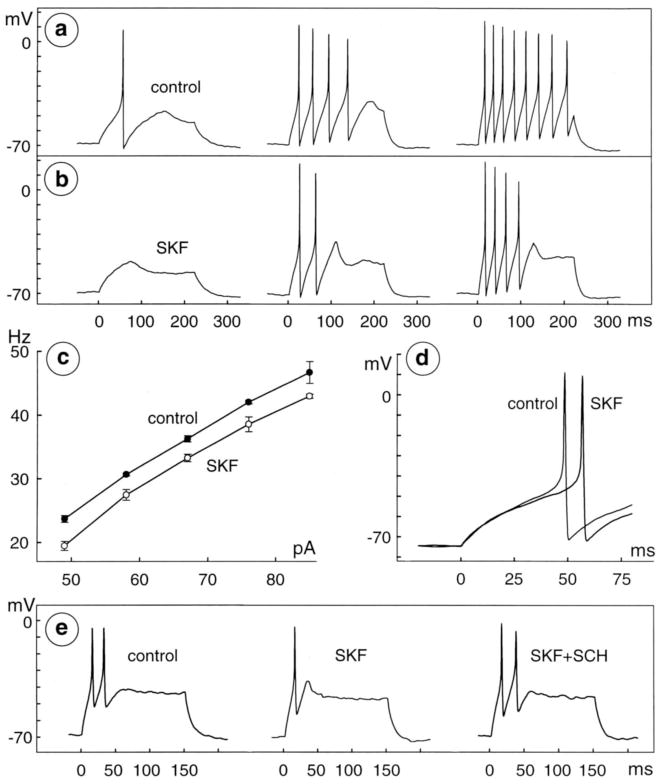
Fig 5.
Reduction of excitability by D1 receptor agonist. a, b, Action potentials were recorded before (a) and during (b) application of 10 μM SKF-38393 in bath solution containing 2.5 mM Ca2+. Spikes fire when cell is depolarized by 225 msec injection of constant current (26, 32, and 35 pA, starting at t=0 msec, in left, middle, and right, respectively). Recordings in each row are displayed with identical 0 mV level; at beginning of traces in both a and b, membrane potential is −71 mV. c, Plot of spike frequency (see Results) versus stimulus intensity (pA). Filled and open circles plot mean frequency of control and test spikes, respectively. Error bars indicate SEM. Spike frequencies were not calculated at stimulus intensities that elicited only one spike. d, Difference in latency of first spike elicited by injection of 29 pA before and during application of 10 μM SKF-38393 (traces labeled control and SKF, respectively, superimposed at fast sweep speed). Data in a–d are from one cell. e, Block of SKF-38393 response (different cell than a–d) by D1 receptor antagonist. Spikes were elicited by 150 msec injection of constant current (55 pA) in control solution (left), 3 μM SKF-38393 (middle), and 3 μM SKF-38393 plus 10 μM SCH-23390 (right). These solutions contained 0.2 mM Ca2+ plus 2.4 mM Mg2+ (compare Fig. 6). Recordings are displayed with identical 0 mV level; membrane potential at beginning of control trace is −70 mV. SCH-23390 alleviates inhibition by SKF-38393.
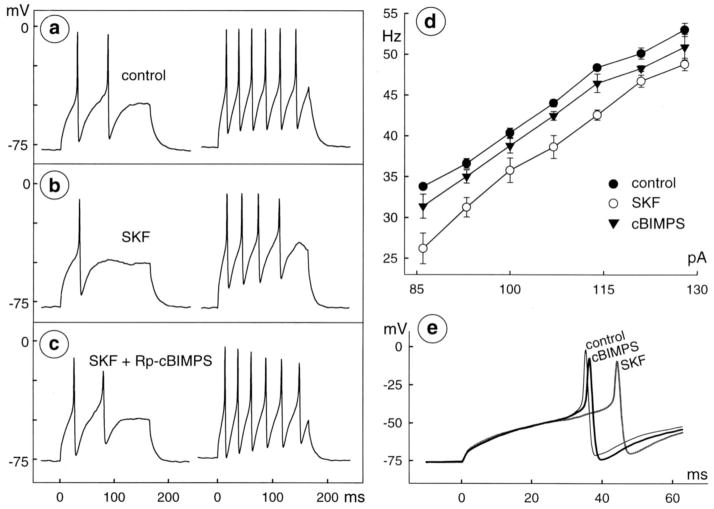
Fig 8.
Reversal of D1 agonist response by protein kinase A inhibitor. Spikes were recorded from a single cell before (a) and during (b) application of 30 μM SKF-38393, and during ( c) subsequent application of a mixture of 30 μM SKF-38393 plus 100 μM Rp-5,6-DCl-cBIMPS. Stimuli are 165 msec, constant-current injections of 72 and 107 pA in left and right, respectively, of a–c. Membrane potential is −78 mV at beginning of left trace in a and b and −77 mV in c. d, Intensity-frequency plot with open and filled circles used as in Figures 5–7 and filled triangles plotting data collected during application of SKF-38393 plus Rp-5,6-DCl-cBIMPS. e, Latency of first spikes elicited by injection of 65 pA in control (thin, black trace), 30 μM SKF-38393 (SKF; thick, dark gray trace), and mixture of 30 μM SKF-38393 plus 100 μM Rp-5,6-DClcBIMPS (cBIMPS; thick, black trace). Records are from same cell as in a–d, superimposed at fast sweep speed. Data were formatted as in Figure 5.
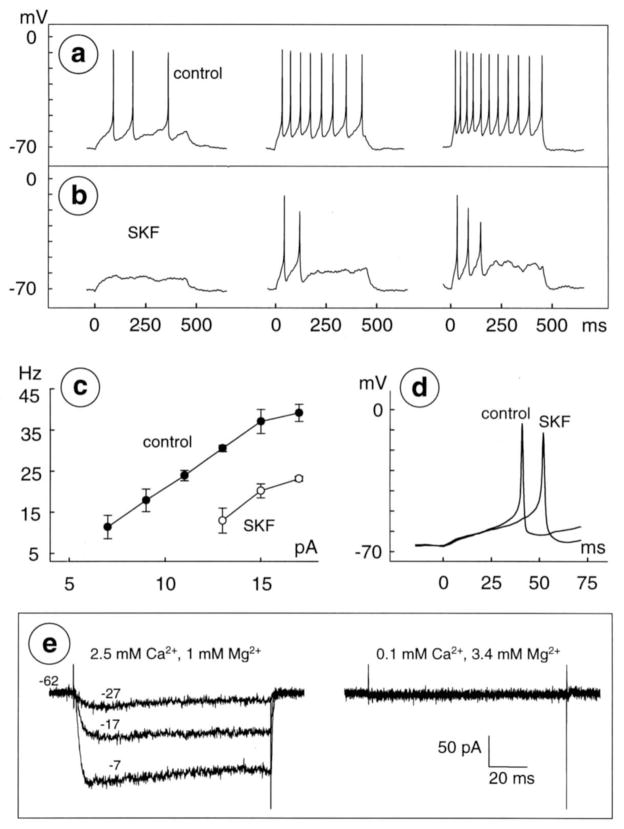
Fig 6.
Reduction of excitability in low-Ca2+ saline. a, b, Spikes recorded before (a) and during (b) application of 10 μM SKF-38393 in bath solution containing 0.1 mM Ca2+ and 2.4 mM Mg2+. Stimuli are 450 msec, constant-current injections of 7,11, and 15 pA, in left, middle, and right of a and b, respectively. Membrane potential at beginning of left trace is −71 mV in both a and b. Intensity-frequency plot ( c) and latency of first spikes elicited by injection of 9 pA before and during application of 10 μM SKF-38393 (superimposed at fast sweep speed in d) are from same cell as in a and b. Data were formatted as in Figure 5. e, Whole-cell current activated by depolarization from a holding potential of −62 mV to test potentials of −27, −17, and −7 mV in control Ringer’s that contained 2.5 mM Ca2+ and 1 mM Mg2+ (left) and in test solution that contained 0.1 mM Ca2+ and 3.4 mM Mg2+ (right). All currents were recorded from same cell (different from that in a–d) and thus show block of inward current on substitution of Mg2+ for most of the bath Ca2+. Similar results were obtained in five cells in test solution containing 0.1 mM Ca2+ and 3.4 mM Mg2+ and in four other cells in test solution containing 0.2 mM Ca2+ and 3.4 mM Mg2+.
Fig 7.
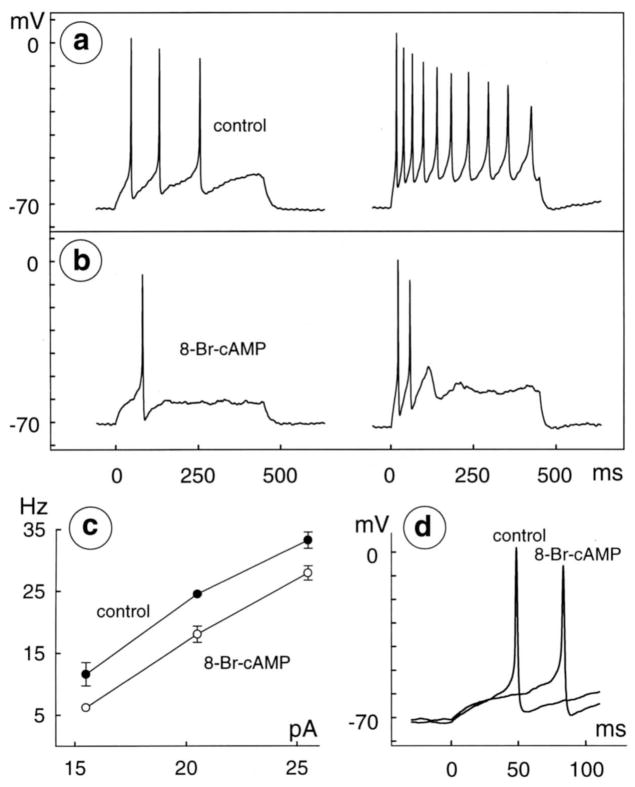
Reduction of excitability by membrane-permeable analog of cAMP. Spikes were recorded before (a) and during (b) application of 100 μM 8-bromo-cAMP in bath solution containing 0.2 mM Ca2+ and 2.4 mM Mg2+. Stimuli are 450 msec, constant-current injections of 15.5 and 30.5 pA in left and right, respectively, of both a and b. Membrane potential is −72 mV at beginning of left trace in a, and −71 mV in b. Intensity– frequency plot ( c) and latency of first spikes elicited by injection of 15.5 pA before and during application of 100 μM 8-bromo-cAMP (superimposed at fast sweep speed in d) from same cell as in a and b. Data were formatted as in Figure 5.
As expected from the suppression of spikes at low stimulus intensities, and the decrease in spike frequency at other intensities, plots of spike frequency versus stimulus current intensity shifted rightward along the frequency axis during responses to SKF-38393 (e.g., Figs. 5, 6, 8) and dopamine (not illustrated). At stimulus current intensities that produced a linear increase in spike frequency, these shifts were parallel. For example, the linear regression slopes of intensity-frequency plots before and during application of 1 μM SKF-38393, were 1.6±0.1 and 1.7±0.2 Hz/pA, respectively (mean±SEM, n=4 cells). As expected from the accommodation noted above, these slopes exceed the control slope measured from spike trains elicited in the same cells by the long depolarizations (1.0±0.2 Hz/pA). However, the control and test slopes calculated from the pairs of spikes were indistinguishable, and consequently, the response of single cells could be described by the vertical displacement between the control and test linear regressions. For example, 1 μM SKF-38393 reduced spike frequency by 7±2 Hz (n=4; Fig. 5c and Table 1). In cells firing at a control rate of 40 Hz, this would correspond to an 18% decrease in spike frequency. D1-type receptor agonists also produced a parallel shift in the intensity-frequency plots of cells that spiked three or more times during step depolarizations in the presence of agonist, when spike frequencies were calculated from the mean of the first two interspike intervals activated by each depolarization (result not illustrated).
Table 1.
Effect of SKF-38393, dopamine, 8-bromo-cAMP, and Rp-5,6-DCl-cBIMPS on spike frequencies and latencies
| Cell # | Ligand | [Ca2+]o | Spike Frequency (Hz) | Latency to First Spike (ms) | ||||
|---|---|---|---|---|---|---|---|---|
| control | test1 | test2 | control | test1 | test2 | |||
| 313074 | SKF | 2.5 | 40 | 32.7 | 31.0 | 40.9 | ||
| 315019 | ” | ” | 30 | 19.8 | 75.8 | 89.6 | ||
| 315035 | ” | ” | 40 | 32.4 | 35.4 | 43.4 | ||
| 316022 | ” | ” | 40 | 37.2 | 39.0 | 55.4 | ||
| 321028 | SKF | 0.1 | 30 | 13.3 | 24.9 | 54.1 | ||
| 329008 | DA | 0.2 | 60 | 49.9 | 15.5 | 20.5 | ||
| 118018 | SKF | 0.1 | 60 | 35.7 | 43.5 | 74.0 | ||
| 404012 | 8-br-cAMP | 0.2 | 40 | 31.1 | 58.4 | 83.9 | ||
| 416035 | ” | ” | 40 | 28.2 | 14.8 | 21.0 | ||
| 410016 | ” | 2.5 | 40 | 34.0 | 28.5 | 31.4 | ||
| 414101 | ” | ” | 40 | 34.9 | 49.3 | 61.5 | ||
| cBIMPS | cBIMPS | |||||||
| 212017 | SKF | 2.5 | 40 | 30.7 | 35.4 | 32.8 | 38.9 | 33.7 |
| 219071 | ” | ” | 40 | 35.8 | 38.8 | 34.3 | 41.8 | 34.3 |
| 209165 | ” | ” | 40 | 30.5 | 35.0 | 20.1 | 28.2 | 20.3 |
Each row describes the spikes recorded in perforated-patch whole-cell mode from a single isolated retinal ganglion cell (n = 14). The test solutions contained SKF-38393 (“SKF”, 1–30 μM), dopamine (“DA”, 0.3–3 μM), 8-bromo-cAMP (“8-br-cAMP”, 100 μM), or Rp-5,6-DCl-cBIMPS (“cBIMPS”, 100–150 μM, applied together with SKF-39393). The external Ca2+ concentrations ([Ca2+]o) used in each recording are listed in mM, and were identical in all solutions applied to a given cell. Control and test spike frequencies are the ordinate values of the points at which a vertical line intersected linear regressions on plots, against stimulus current intensity, of the inverse of time elapsed between the first two spikes elicited by each current injection, in the solutions indicated. The “latency to first spike” is the time elapsed from the beginning of a current injection to the peak of the first spike elicited, in all of the solutions indicated, by the smallest amount of current that elicited spikes in the test solution. Latency values are means of measurements obtained from 2–7 identical current injections into each cell in each solution.
Effects of dopamine receptor activation on spike timing were gauged from the times that elapsed between the onset of current injections and the peak of the first spike elicited, in control versus test solutions, using the smallest current intensity that activated spikes in the test solution. In saline that contained normal (2.5 mM) Ca2+, 1 μM SKF-38393 increased this latency by 27±4% over the control value (45±10 msec, mean±SEM, n=4; Table 1). Dopamine agonists produced smaller increases in the latency of spikes elicited by larger stimulus current intensities, and at the largest stimuli used (45–75 pA), no change in latency was noticeable. These agonists thus reduced spike number and frequency at all of the stimulus intensities we used, but they increased spike latency most noticeably during smaller current injections (e.g., Fig. 5d). As discussed below, these changes in spike frequency and firing pattern resemble effects of background light, whereas the increase in latency at low and moderate stimulus intensities does not (see Discussion).
All of the above effects occurred without changes in resting potential (Figs. 5–8). Inhibition was reversible, and appeared to be mediated by a D1-type dopamine receptor, because these effects were reversed either by washing away the agonists with agonist-free bath solution (result not illustrated), or by co-application of 10 μM SCH-23390 (e.g., Fig. 5e). Effects of SKF-38393, and of dopamine, were observed within ≤1 min of the onset of application, and thereafter, for as long as either agonist was applied (≤ 14 min). Recovery from inhibition occurred within 1–3 min of microperfusing control bath solution, or a mixture of antagonists and agonists, onto cells.
Dopamine receptor activation did not increase spike firing in any ganglion cells that we have recorded from. However, the number of cells that we have recorded from successfully (i.e., in which our recordings were stable enough to analyze for this study; n=14) is smaller than the number of morphological subtypes of ganglion cells that have been identified in various species. If one assumes that the goldfish retina contains 15 different types of retinal ganglion cells (Hitchcock and Easter, 1986), that each type is either inhibited by dopamine or not, and that each type is equally likely to have been recorded from, then our having found that 14 out of 14 cells were inhibited by dopamine indicates, with a probability value of 0.044, that at least 13 of these 15 types of ganglion cells are inhibited by dopamine. At the same time, our results do not exclude the possibility that some types of ganglion cells that we have not recorded from might not respond to dopamine, or might be excited by dopamine.
Ca2+ current blockade does not prevent inhibition by dopamine
Dopamine has been found to decrease spike frequency, and increase spike latency, in cat retinal ganglion cells in situ (Straschill and Perwein, 1969) and in isolated turtle retinal ganglion cells (Liu and Lasater, 1994). These results were obtained either in unperfused eyes, or in Ringer solution that contained a normal Ca2+ concentration. It is possible that dopamine alters spiking by affecting Ca2+ influx into these cells (Liu and Lasater, 1994), especially because spike frequency is reduced in retinal ganglion cells by other agents that reduce voltage-gated Ca2+ currents, including GABAB receptor agonists, Co2+ and Cd2+, and the Conus toxins ω-MVIIC and ω-GVIA (Liu and Lasater, 1994; Zhang et al., 1997; Rothe et al., 1999). We therefore tested whether D1-type receptor activation inhibits spiking after Mg2+ was substituted for nearly all of the Ca2+ in the bath solution (Fig. 6). This treatment blocks voltage-gated Ca2+ currents in ganglion cells (Fig. 6e), yet retains enough extracellular Ca2+ to avoid undesired effects of Ca2+-free solutions (e.g. Almers et al., 1984).
Each of the effects shown in Fig. 5a–d were observed in bath saline that contained 2.4 mM Mg2+, and either 0.1 or 0.2 mM Ca2+. SKF-38393 (10–20 μM; n=2) and dopamine (0.3 μM; n=1) abolished spikes produced by small depolarizations (Figs. 6a, b), truncated repetitive spiking (Fig. 6b), reduced spike frequency by 17±4 Hz (Fig. 6c and Table 1), and increased spike latency by 109±58% over the control value (32±8 msec; e.g., Fig. 6d and Table 1).
8-bromo-cAMP reduces retinal ganglion cell excitability
Having found that dopamine receptor stimulation augments the concentration of cAMP inside ganglion cells (Figs. 3, 4) and reduces their excitability (Figs. 5, 6, 8), we next tested whether 8-bromo-cAMP reduced ganglion cell excitability. This membrane-permeable cAMP analog produced the same four effects on excitability that were seen during D1-type agonist applications. In saline that contained either 0.2 mM Ca2+ (n=2) or 2.4 mM Ca2+ (n=2), 100 μM 8-bromo-cAMP abolished spikes produced by small depolarizations (Figs. 7b), truncated repetitive spiking (Fig. 7b), reduced spike frequency by 8±5 Hz (Fig. 7c and Table 1), and increased spike latency by 30±8% over the control value (38±10 msec; Fig. 7d and Table 1). The decrease in spike frequency produced by 100 μM 8-bromo-cAMP is similar to that produced by all of the concentrations of SKF-38393 that we tested (1–30 μM). Because the above results show that both D1-type dopamine receptor agonists and 8-bromo cAMP inhibit ganglion cells, we did not test whether 8-bromo-cAMP or cAMP activate a cyclic nucleotide-gated inward current in ganglion cells (Ahmad et al., 1994).
Rp-5,6-DCl-cBIMPS blocks inhibition by D1 receptor activation
Dopamine and cAMP modulate ion currents in various cells, via activation of protein kinase A (PKA). We therefore tested whether a PKA inhibitor could block ganglion cell responses to D1-type receptor agonists. Figure 8 shows that the reduction of spike frequency, curtailment of repetitive spiking, and increase in spike latency by 30 μM SKF-38393 were all counteracted by a membrane-permeable PKA inhibitor (Rp-5,6-DCl-cBIMPS, 100 μM; Cantrell et al., 1997). Similar effects were observed in all of three cells tested (Table 1). SKF-38393 reduced spike frequency by 8±2 Hz. During subsequent application of a mixture of SKF-38393 and Rp-5,6-DCl-cBIMPS, spike frequency increased until it differed from the control rate by 4±1 Hz (Figs. 8a–d and Table 1). During the same applications of SKF-38393, spike latency increased to 27±7% over control values, whereas during the application of SKF-38393 together with Rp-5,6-DCl-cBIMPS, spike latency was only 1±0.8% longer than the control values (Fig. 8e and Table 1). These recordings also show that Rp-5,6-DCl-cBIMPS counteracted the effect of D1-receptor activation on repetitive spiking, restoring the number of spikes elicited by a given current injection, to control levels (Figs. 8a, b, c).
We did not test for effects of PKA inhibitors by inclusion in the pipette solution during ruptured-patch recordings, because cells were unresponsive to D1 receptor agonists in this recording configuration (cf., Horn and Marty, 1988; Vargas et al., 1999).
DISCUSSION
We have combined immunostaining and spike recording to examine how light sensitivity of the retina’s output neurons is modulated by background illumination. Our results provide the first evidence that light-stimulated release of dopamine raises retinal ganglion cell cAMP levels in situ, and that dopaminergic inhibition of these cells can be reversed by a protein kinase A inhibitor. These results imply that ganglion cell spike generation can be modulated by adapting light, by dopaminergic activation of a protein kinase A. Previous studies showed that light adaptation occurs in photoreceptors (e.g, Baylor and Hodgkin, 1974), bipolar cells (e.g., Ashmore and Falk, 1980), and horizontal cells (e.g., Piccolino et al., 1984), i.e., in both tiers of neurons that are distal to ganglion cells. Combining these observations, with ours, indicates that all three layers of neurons in the retina are equipped with mechanisms for adaptation to ambient light. Below, we discuss how dopaminergic inhibition of ganglion cells may be triggered in situ, and how this inhibition compares with that induced by adapting lights.
Light- and dopamine-modulated excitability
We found that ganglion cell cAMP levels are elevated in fish kept in light during daytime, but not in fish collected in darkness at night; that these cAMP levels increase if retinas are illuminated at night, and decrease if retinas are darkened during daytime; that exogenous dopamine can elevate ganglion cell cAMP levels in dark-adapted retinas and that this increase can be blocked by SCH-23390; and that SCH-23390 reduces ganglion cell cAMP levels in light-adapted retinas. The simplest explanation of these results is that ganglion cell cAMP levels can be increased by light-stimulated dopamine release, regardless of the time of day. In the species studied here, this dopamine would be released by a type of interplexiform cell (Kalloniatis and Marc, 1990).
How does dopamine inhibit spikes in retinal ganglion cells? Our observations that endogenous and exogenous dopamine increase cAMP levels in retinal ganglion cells, that ganglion cell excitability is reduced by dopamine, SKF-38393, and 8-bromo-cAMP, and that the inhibitory effect of SKF-38393 is counteracted by Rp-5,6-DCl-cBIMPS, altogether indicate that spiking is inhibited by activation of a D1-type receptor-coupled, cAMP-dependent protein kinase (protein kinase A). It is possible that this effect is exerted on voltage-gated Na+ currents and /or on voltage-gated K+ currents, because similar results were obtained in media that support or block voltage-gated Ca2+ currents, inward rectification activates at membrane potentials more negative than those traversed here (Tabata and Ishida, 1996), and the resting Cl− permeability of ganglion cells is controlled by protein kinase C (Tabata and Ishida, 1999). We have not identified which voltage-gated current properties are modulated by dopamine, or how protein kinase A activation modulates currents. However, our results show that dopamine can reduce retinal ganglion cell excitability independently of effects on voltage-gated Ca2+ currents (cf., Liu and Lasater, 1994).
Our results also show that dopaminergic inhibition of ganglion cells does not require modulation of the release of, or response to, neurotransmitters. Exogenous dopamine inhibits spontaneous and light-evoked spike firing in ganglion cells in situ, and it can also inhibit spikes elicited by exogenous glutamate (Straschill and Perwein, 1969; Thier and Alder, 1984). This implies that even if dopamine augments the synaptic output of bipolar cells (Heidelberger and Matthews, 1994), the concomitant reduction of retinal ganglion cell excitability would tend to lower spike output.
Our results do not imply that only dopamine modulates ganglion cell excitability or that a single mechanism accounts for network adaptation. For example, dopamine reduces light responses of some on- and off-center bipolar cells (Shiells and Falk, 1985; Hare and Owen, 1995), dopamine and other substances alter the transmission of rod and cone signals (e.g., Witkovsky et al., 1988; Hampson et al., 1992; Mills and Massey, 1995; Wang and Mangel, 1996; Krizaj et al., 1998; Manglapus et al., 1999), fast as well as slow adaptation to a variety of stimuli have been demonstrated in ganglion cells (e.g., Enroth-Cugell and Shapley, 1973; Green et al., 1975; Yeh et al., 1996; Smirnakis et al., 1997; Kim and Rieke, 2001), and voltage-induced changes in spike-generation have been shown to underlie temporal contrast adaptation in ganglion cells (Kim and Rieke 2001).
Adaptation
Without knowing the effect of dopamine on neurotransmitter release from bipolar and amacrine cells onto ganglion cells, or whether dopamine modulates ganglion cell responses to these transmitters, it is difficult to account in detail for ganglion cell light responses under conditions that induce network adaptation. However, if D1 receptor agonists and 8-bromo-cAMP produce effects in ganglion cells that resemble those of background illumination, one would infer that these effects either add to, or outweigh, those on other cells. How do these compare? First, SKF-38393, dopamine, and 8-bromo-cAMP raise spike threshold, because larger currents are required to elicit individual spikes, or a given spike frequency, during their application. Likewise, background illumination reduces the number and frequency of spikes that are elicited at different light intensities (Sakmann and Creutzfeld, 1969). This transforms ganglion cells from luminance detectors into contrast detectors (Barlow and Levick, 1969; Sakmann and Creutzfeld, 1969). Three results indicate that this occurs, to some extent, without changes in ganglion cell sensitivity to changes in stimulus current or synaptic input. We found that D1-type receptor activation and 8-bromo-cAMP produce parallel, right-ward shifts in plots of spike frequency versus stimulus current intensity, and likewise, exogenous dopamine (Jensen and Daw, 1986) and background illumination (Sakmann and Creutzfeld, 1969) produce parallel, right-ward shifts in plots of spike frequency versus light intensity.
Second, D1-type receptor activation reduced the ability of all of the ganglion cells we recorded from to spike repeatedly during prolonged depolarizations (Figs. 5, 6, 8). Likewise, background light truncates the spike trains of dark-adapted ganglion cells, transforming sustained cells into transient cells (Yoon, 1972; Jakiela et al., 1976). Thus, background light and D1-type receptor activation filter ganglion cell responses to sustained stimuli. This transforms ganglion cells from low-pass filters (where spiking is limited by the maximum firing rate) to band-pass filters (where spiking is damped at low and high stimulus frequencies). Our results suggest that this transformation can occur in a relatively simple and unconstrained way. First, it does not require a change in resting potential as do other mechanisms (Jahnsen and Llinás, 1984; Tabata and Ishida, 1996). Second, while this transformation could occur along with changes in bipolar cell light response and transmitter release kinetics (see Richter and Simon, 1975; Kaneko et al., 1979; Diamond and Copenhagen, 1993; Awatramani and Slaughter, 2000) and recruitment of inhibitory receptive field surround input (Barlow et al., 1957; Barlow and Levick, 1969), dopamine could initiate and sustain this transformation without acting through bipolar, horizontal, or amacrine cells.
Third, light augmented cAMP levels in all ganglion cells in a given field of view (Figs. 1–4). This is consistent with the presence of D1-receptor-like immunoreactivity in all ganglion cell somata in the species examined here (Mora-Ferrer et al., 1999), presence of a dopamine-releasing type of interneuron throughout the retina (Kalloniatis and Marc, 1990), and both paracrine and synaptic transmission (Piccolino et al., 1984; Yazulla and Zucker, 1988; Witkovsky et al., 1993). The staining pattern we have seen is also consistent with the ability of dopamine to right-shift the intensity-response curves of both ON- and OFF-center types of ganglion cells (Jensen and Daw, 1986), and the presence of these cell types throughout the retina (Wässle and Boycott, 1991).
Fourth, ganglion cell cAMP levels were low at night and elevated during the day. This suggests that ganglion cell excitability might wax and wane in a circadian rhythm. Although we have not tested this possibility directly, reflex measurements show that goldfish are more light sensitive at night than during day (Bassi and Powers, 1987). Moreover, dopamine release can be initiated before dawn by a circadian oscillator (e.g., Kolbinger et al., 1990). This does not appear to be unique to fish, because mammalian light sensitivity is lower in daytime than at night (Brandenburg et al., 1983), dopamine release is circadian (Dubocovich, 1983), and we have found that anti-cAMP antisera immunostain ganglion cell somata in light-adapted rat retinas (Vaquero and Ishida, unpublished observations). It should be noted that our experiments were not designed to test if cAMP levels were elevated in daylight because they increased in response to the light of dawn, or in anticipation of dawn. However, in retinas where dopamine release continues during the day, network adaptation can be elicited by illumination at night, and dopamine release can be decreased by darkness imposed during the day (Kolbinger et al., 1990; Wang and Mangel, 1996; Manglapus et al., 1999). Our having found that ganglion cell cAMP levels can be elevated by light at night, and reduced by darkness during day, are thus not at odds with circadian dopamine release.
In summary, we have found that retinal ganglion cells are affected by D1-type receptor activation in ways that mimic background illumination. Our results are consistent with recordings from ganglion cells in intact retinas and with behavioral effects of dopamine depletion. However, unlike background illumination (Pickering, 1968; Enroth-Cugell and Lennie, 1975), dopamine, SKF-38393, and 8-bromo-cAMP increased spike latency (Figs. 5–8). We suppose that the spike latency decrease produced by background illumination is due, at least in part, to acceleration of photoreceptor and bipolar cell light responses and transmitter release (Baylor and Hodgkin, 1974; Richter and Simon, 1975; Diamond and Copenhagen, 1993; Donner et al., 1995). Examining this aspect of network adaptation, and others, will require recordings from ganglion cells with intact synaptic inputs and adaptable light responses.
Acknowledgments
This work was supported by NIH grant EY08120 (to ATI) and NEI Core Grant P30 EY12576. The authors thank W.G. Owen, M. Piccolino, and K.H. Britten for comments on the manuscript; A.J. Sillman for use of infra-red image-converting goggles; S.C. Lee for performing the recordings exemplified in Fig. 5e; R.A. Fontanilla for assistance in confocal imaging; and K.E. Munckton for assistance with some of the data analysis.
References
- Almers W, McCleskey EW, Palade PT. A non-selective cation conductance in frog muscle membrane blocked by micromolar external Ca2+ ions. J Physiol (Lond) 1984;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF, Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol (Lond) 1980;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci. 2000;20:7087–7095. doi: 10.1523/JNEUROSCI.20-18-07087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol (Lond) 1969;202:699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK. Daily fluctuations in the detectability of dim lights by humans. Physiol Behav. 1986;38:871–877. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK. Circadian rhythm in goldfish visual sensitivity. Invest Ophthalmol Vis Sci. 1987;28:1811–1815. [PubMed] [Google Scholar]
- Baylor DA, Hodgkin AL. Changes in time scale and sensitivity of turtle photoreceptors. J Physiol (Lond) 1974;242:729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Makman MH. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3′:5′-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci USA. 1972;69:539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Smith RD, Goldin AL, Scheuer T, Catterall WA. Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel α subunit. J Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI. Increased levels of 3′,5′-cyclic adenosine monophosphate induced by cobaltous ion or 3-isobutylmethylxanthine in the incubated mouse retina: Evidence concerning location and response to ions and light. J Neurochem. 1982;38:781–796. doi: 10.1111/j.1471-4159.1982.tb08699.x. [DOI] [PubMed] [Google Scholar]
- Dearry A, Burnside B. Light-induced dopamine release from teleost retinas act as a light-adaptive signal to the retinal pigment epithelium. J Neurochem. 1989;53:870–878. doi: 10.1111/j.1471-4159.1989.tb11785.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Farber DB, Souza DW, Chase DG, Lolley RN. Cyclic nucleotides of cone-dominant retinas. Invest Ophthalmol Vis Sci. 1981;20:24–31. [PubMed] [Google Scholar]
- Glickman RD, Adolph AR, Dowling JE. Inner plexiform circuits in the carp retina: effects of cholinergic agonists, GABA, and substance P on the ganglion cells. Brain Res. 1982;234:81–99. doi: 10.1016/0006-8993(82)90474-7. [DOI] [PubMed] [Google Scholar]
- Green DG, Dowling JE, Siegal IM, Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol. 1975;65:483–502. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggendahl J, Malmfors T. Identification and cellular localization of the catecholamines in the retina and the choroid of the rabbit. Acta Physiol Scand. 1965;64:58–66. doi: 10.1111/j.1748-1716.1965.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Hampson ECGM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalina retina. J Neurosci. 1992;12:4991–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA, Owen WG. Similar effects of carbachol and dopamine on neurons in the distal retina of the tiger salamander. Vis Neurosci. 1995;12:443–455. doi: 10.1017/s0952523800008348. [DOI] [PubMed] [Google Scholar]
- Hedden WL, Dowling JE. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond. 1978;201B:27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Mattews G. Dopamine enhances Ca2+ responses in synaptic terminals of retinal bipolar neurons. NeuroReport. 1994;5:729–732. doi: 10.1097/00001756-199402000-00018. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Ishida AT. Voltage-gated Na+ current availability after step- and spike-shaped conditioning depolarizations of retinal ganglion cells. Pflügers Arch. 1998;436:497–508. doi: 10.1007/s004240050664. [DOI] [PubMed] [Google Scholar]
- Ishida AT, Cohen BN. GABA-activated whole-cell currents in isolated retinal ganglion cells. J Neurophysiol. 1988;60:381–396. doi: 10.1152/jn.1988.60.2.381. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: An in vitro study. J Physiol (Lond) 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RJ, Daw NW. Effects of dopamine and its agonists and antagonists on the receptive field properties of ganglion cells in the rabbit retina. Neurosci. 1986;17:837–855. doi: 10.1016/0306-4522(86)90049-7. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Marc RE. Interplexiform cells of the goldfish retina. J Comp Neurol. 1990;297:340–358. doi: 10.1002/cne.902970303. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Famiglietti EV, Jr, Tachibana M. Physiological and morphological identification of signal pathways in the carp retina. In: Otsuka M, Hall Z, editors. Neurobiology of chemical transmission. New York: Wiley; 1979. pp. 235–251. [Google Scholar]
- Kolbinger W, Kohler K, Oetting H, Weiler R. Endogenous dopamine and cyclic events in the fish retina, I: HPLC assay of total content, release, and metabolic turnover during different light/dark cycles. Vis Neurosci. 1990;5:143–149. doi: 10.1017/s0952523800000183. [DOI] [PubMed] [Google Scholar]
- Kramer SG. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol. 1971;10:438–452. [PubMed] [Google Scholar]
- Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- Lin Z-S, Yazulla S. Depletion of retinal dopamine increases brightness perception in goldfish. Vis Neurosci. 1994;11:683–693. doi: 10.1017/s0952523800002996. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Calcium currents in turtle retinal ganglion cells. II. Dopamine modulation via a cyclic AMP-dependent mechanism. J Neurophysiol. 1994;71:743–752. doi: 10.1152/jn.1994.71.2.743. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Lawrence JE, Valentino TL. Desensitizing glutamate receptors shape excitatory synaptic inputs to tiger salamander retinal ganglion cells. J Neurosci. 1995;15:6189–6199. doi: 10.1523/JNEUROSCI.15-09-06189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1999;19:4132–4141. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Liu W-L, Kalloniatis M, Raiguel SF, Van Haesendonck E. Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci. 1990;10:4006–4034. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by All amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Mora-Ferrer C, Dabin I, Stell WK. In situ hybridization of retinal dopamine D1 receptor in goldfish. Invest Ophthal Vis Sci. 1996;37:951S. [Google Scholar]
- Mora-Ferrer C, Yazulla S, Studholme KM, Haak-Frendscho M. Dopamine D1-receptor immunolocalization in goldfish retina. J Comp Neurol. 1999;411:705–714. doi: 10.1002/(sici)1096-9861(19990906)411:4<705::aid-cne14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Orr HT, Lowry OH, Cohen AI, Ferrendelli JA. Distribution of 3′:5′-cyclic AMP and 3′:5′-cyclic GMP in rabbit retina in vivo: Selective effects of dark and light adaptation and ischemia. Proc Natl Acad Sci USA. 1976;73:4442–4445. doi: 10.1073/pnas.73.12.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′:5′-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering SG. The extremely long latency response from on-off retinal ganglion cells: relationship to dark adaptation. Vis Res. 1968;8:383–387. doi: 10.1016/0042-6989(68)90107-7. [DOI] [PubMed] [Google Scholar]
- Pirenne MH. Some aspects of the sensitivity of the eye. Ann NY Acad Sci. 1958;74:377–384. doi: 10.1111/j.1749-6632.1958.tb39559.x. [DOI] [PubMed] [Google Scholar]
- Richter A, Simon EJ. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol (Lond) 1975;248:317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe T, Jüttner R, Bähring R, Grantyn R. Ion conductances related to development of repetitive firing in mouse retinal ganglion neurons in situ. J Neurobiol. 1999;38:191–206. [PubMed] [Google Scholar]
- Sakmann B, Creutzfeld O. Scotopic and mesopic light adaptation in the cat’s retina. Pflügers Arch. 1969;313:168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Lam DMK. The uptake and release of [3H]dopamine in the goldfish retina. J Neurochem. 1979;32:1269–1277. doi: 10.1111/j.1471-4159.1979.tb11054.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Lledo P-M, Vincent J-D. Dopamine D1 receptor modulates voltage-gated sodium current in rat striatal neurones through a protein kinase A. J Physiol (Lond) 1995;483:95–107. doi: 10.1113/jphysiol.1995.sp020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Enroth-Cugell C. Visual adaptation and retinal gain controls. Progr Ret Res. 1984;3:263–346. [Google Scholar]
- Shiells RA, Falk G. Dopamine hyperpolarizes and reduces the light responses of rod on-center bipolar cells in the retina of the dogfish. Neurosci Lett. 1985;55:331–336. doi: 10.1016/0304-3940(85)90457-4. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Parker CW, Kipnis DM. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- Straschill M, Perwein J. The inhibition of retinal ganglion cells by catecholamines and γ-aminobutyric acid. Pflügers Arch. 1969;312:45–54. doi: 10.1007/BF00588530. [DOI] [PubMed] [Google Scholar]
- Tabata T, Ishida AT. Transient and sustained depolarization of retinal ganglion cells by Ih. J Neurophysiol. 1996;75:1932–1944. doi: 10.1152/jn.1996.75.5.1932. [DOI] [PubMed] [Google Scholar]
- Tabata T, Ishida AT. A zinc-dependent Cl− current in neuronal somata. J Neurosci. 1999;19:5195–5204. doi: 10.1523/JNEUROSCI.19-13-05195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier P, Alder V. Action of iontophoretically applied dopamine on cat retinal ganglion cells. Brain Res. 1984;292:109–121. doi: 10.1016/0006-8993(84)90895-3. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, Ishida AT. Endogenous dopamine increases cAMP levels in fish retinal ganglion cells in daylight, but not at night. Investig Ophthal Vis Sci. 2000;41:936. [Google Scholar]
- Wagner H-J, Behrens UD. Microanatomy of the dopaminergic system in the rainbow trout retina. Vis Res. 1993;33:1345–1358. doi: 10.1016/0042-6989(93)90042-u. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Nicholson C, Rice ME, Bohmaker K, Meller E. Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proc. Natl. Acad. Sci. USA. 1993;90:5567–5671. doi: 10.1073/pnas.90.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Yamada M, Saito T. Effects of dopamine on bipolar cells in the carp retina. Biomedical Research, Supplement. 1988;9:125–130. [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci. 1988;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]
- Yoon M. Influence of adaptation level on response pattern and sensitivity of ganglion cells in the cat’s retina. J Physiol. 1972;221:93–104. doi: 10.1113/jphysiol.1972.sp009741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Anderson K, Sakaguchi H, Flannery J, FitzGerald P, Ishida AT. Voltage-gated Na+ channel EOIII-segment-like immunoreactivity in fish retinal ganglion cells. Vis Neurosci. 2000;17:647–655. doi: 10.1017/s0952523800174139. [DOI] [PubMed] [Google Scholar]
- Young LHY, Dowling JE. Localization of cyclic adenosine monophosphate in the teleost retina: effects of dopamine and prolonged darkness. Brain Res. 1989;504:57–63. doi: 10.1016/0006-8993(89)91597-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shen W, Slaughter MM. Two metabotropic γ-aminobutyric acid receptors differentially modulate calcium currents in retinal ganglion cells. J Gen Physiol. 1997;110:45–58. doi: 10.1085/jgp.110.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]