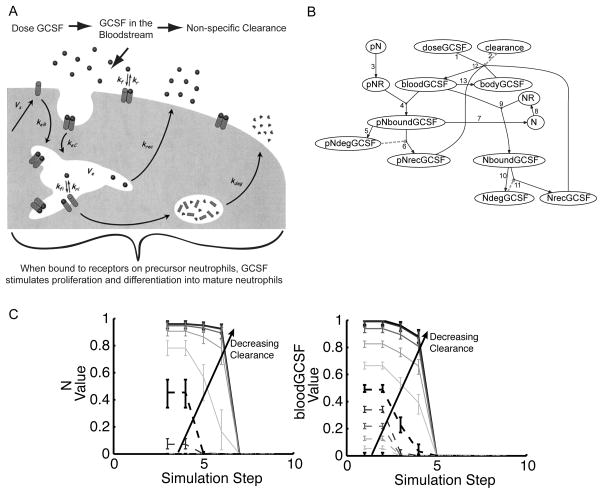
Figure 5. Development of logic model of G-CSF administration.
A: Depiction of G-CSF pharmacokinetics at the tissue, cellular, and molecular level. adapted from [23].
B: Logic model based on 5A. All transfer functions have default parameters g = 1; n = 3; and EC50 = 0.5. Arrow labels indicate the following steps of the pharmacokinetics of the molecule: (1) When G-CSF is administered intravenously (doseGCSF), it enters the bloodstream where it is subject to (2) nonspecific clearance (clearance). (3) Precursor neutrophils (pN) possess receptors (pNR), which (4) bind G-CSF in the blood (pNboundGCSF). (5) Bound G-CSF can be degraded (pNdegGCSF), and (6) what is not degraded is recycled back into the bloodstream (pNrecGCSF). (7) Bound G-CSF also stimulates proliferation and differentiation into mature neutrophils (N). (8) Mature neutrophils possess receptors (NR) that can (9) bind G-CSF (NboundGCSF). Bound G-CSF is then (10) degraded (NdegGCSF) or (11) recycled (NrecGCSF). (12) Value of G-CSF in the blood (bloodGCSF) is limited by the dose, clearance, and amount recycled. (13) An additional species bodyGCSF represents the exchange of G-CSF from the blood to the body cavity and is necessary in the logic model to ensure that the bloodGCSF node is also limited by its own value.
C: The G-CSF logic model was simulated under non-limiting precursor neutrophils and dose conditions (pN = 1 and doseGCSF = 1) with multiple levels of clearance (0, 0.1, 0.2, etc.). Median value of the neutrophil and G-CSF levels in the blood nodes (N and bloodGCSF) were plotted as a function of simulation step, with error bars indicating the first and third quartile of predictions of 100 models with normally distributed noise with a standard deviation of five percent added to the transfer function parameters. As levels of clearance decreased, maximal values of N and bloodGCSF increased as well as the number of simulation steps until the species values decreased to zero. Further analysis indicated adding noise with a standard deviation of up to 25 percent led to identical conclusions for all results.