Figure 2.
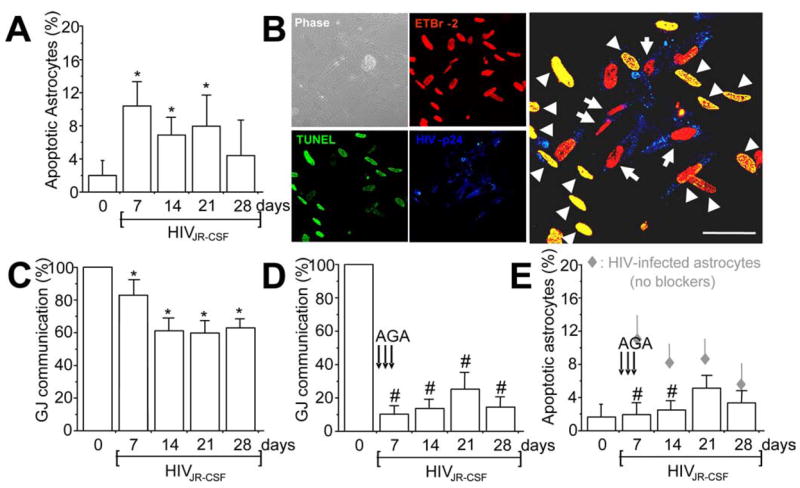
HIV infection of astrocytes induces apoptosis in uninfected astrocytes by a gap junction-dependent mechanism. Data obtained with infection of astrocytes with HIVJR-CSF, a virus isolated from the CSF, are presented in the figure. The result for the other two viruses used are in the supplemental material (available at www.jneurosci.org). A, Quantification of apoptosis was performed by double labeling with TUNEL and GFAP staining. HIV infection of astrocyte cultures with HIVJR-CSF resulted in significantly increased apoptosis after 7, 14, and 21 d after infection (*p < 0.005; n = 15). B, Immunofluorescence staining and confocal analyses of the HIV-infected cultures 7 d after infection for ETBr-2 (nuclei labeling, red), TUNEL (apoptosis, green, FITC), and p24 staining (HIV, blue, Alexa350), shows that most p24-positive astrocytes were not apoptotic (red nuclei in the merge picture, 68 ± 8% of HIV-infected astrocytes survive HIV infection) compared with uninfected astrocytes in contact with HIV-infected cells that are apoptotic (yellow nuclear staining, 100% cells in contact with HIV-infected astrocytes were apoptotic). The monolayer of cells can be observed in the phase picture (Phase). HIV-infected astrocytes only corresponded to 4.7 ± 2.8% of the total number of astrocytes in the culture. Arrows indicate HIV-infected astrocytes that are not apoptotic, and arrowheads indicate uninfected apoptotic astrocytes. Scale bar, 40 μm. C, Whether HIVJR-CSF infection alters GJ communication was evaluated by a scrape loading technique. HIV infection reduced GJ communication ~20–40% compared with uninfected astrocytes (100%), but GJs still remained functional (*p < 0.003; n = 11). D, To evaluate whether GJ communication participates in apoptosis of uninfected astrocytes in contact with HIV-infected astrocytes, gap junction blockers were used. Addition of AGA (50 μM) or octanol (500 μM) after 1, 2, and 3 d after infection reduced GJ communication by ~70% compared with HIV-infected astrocytes cultures without the blockers. Similar results were found using octanol (data not shown). #p < 0.002, n = 17, AGA with respect to GJ communication shown in C. E, Blocking gap junction channels with AGA treatment resulted in significant reduction in astrocyte apoptosis mediated through HIV-infected cells (#p < 0.003; n = 17). These results demonstrate that GJ communications mediate transfer of proapoptotic signals between HIV-infected astrocytes and uninfected astrocytes that form GJs with infected cells. Gray diamonds represent the values of apoptosis shown by HIV-infected cultures without AGA (results shown in A), for comparison of apoptosis levels with AGA (E, #p < 0.0002; n = 17).
