Figure 5.
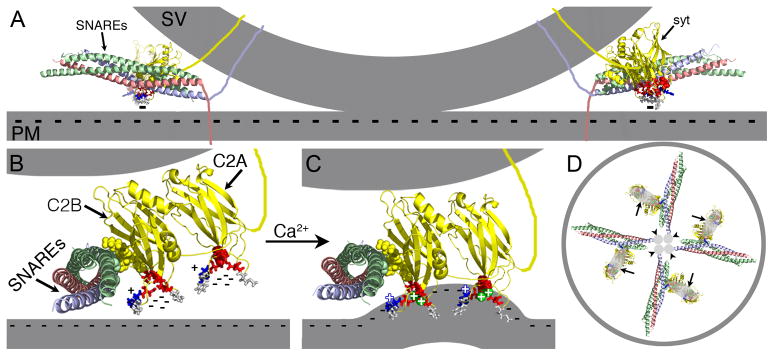
Ca2+ binding by C2A is an essential component of the electrostatic switch. The crystal structure of the core complex [PDB file 1SFC, containing syntaxin (red), SNAP-25 (green), and VAMP/synaptobrevin (blue)], the NMR structures of the C2A (PDB file 1BYN) and C2B (PDB file 1K5W) domains of synaptotagmin (yellow), and Ca2+ (green spheres “+”) are shown to scale using PyMOL. The membranes, the transmembrane domains, and the link between C2A and C2B were added in Adobe Photoshop. A) Cross section of a docked vesicle showing two SNARE complexes (SNAREs) and their associated synaptotagmin molecules (syt). B) One syt/SNARE complex viewed from the site of vesicle/presynaptic membrane apposition. A Ca2+-independent docking/priming interaction between the C2B polylysine motif (yellow, space-filled residues) and SNAP-25 [green, space-filled residues, (Rickman et al., 2004; Loewen et al., 2006b)] holds the C2B Ca2+-binding site immediately adjacent to the presynaptic membrane with the C2A Ca2+-binding site further removed. In the absence of Ca2+, the conserved aspartate residues (red residues: sytC2A-D2, space-filled; the rest as sticks) within the pockets create a high concentration of negative charge (cluster of “−”s) resulting in electrostatic repulsion of the presynaptic membrane that prevents any membrane interactions by the tips of the C2 domains. C) Upon Ca2+ binding, the electrostatic repulsion of the pockets is neutralized thereby initiating the electrostatic switch: a strong attraction of the negatively-charged membrane by the bound Ca2+ (green spheres “+”) and the basic residues at the tips of Ca2+-binding pockets (blue stick residues “+”). Insertion of the hydrophobic residues (grey stick residues) at the tips of the C2 domains into the core of the presynaptic membrane then triggers fusion by promoting a local Ca2+-dependent positive curvature of the plasma membrane (Martens et al., 2007; Hui et al., 2009; Paddock et al., 2011). The C2 domain interactions with the membrane likely pull the synaptic vesicle (upper grey membrane) toward the presynaptic membrane (lower grey membrane). The Ca2+-induced increase in positive charge at the end of the C2B domain also likely increases the strength of the electrostatic interaction between the C2B polylysine motif and the SNARE complex, resulting in simultaneous binding of the SNARE complex and the presynaptic membrane (Davis et al., 1999; Bhalla et al., 2006; Loewen et al., 2006a; Dai et al., 2007). D) Membrane penetration by multiple synaptotagmins (large grey ovals, arrows) would pull the plasma membrane toward the vesicle in a ring around the SNARE transmembrane domains (small grey circles, arrowheads) facilitating fusion.
