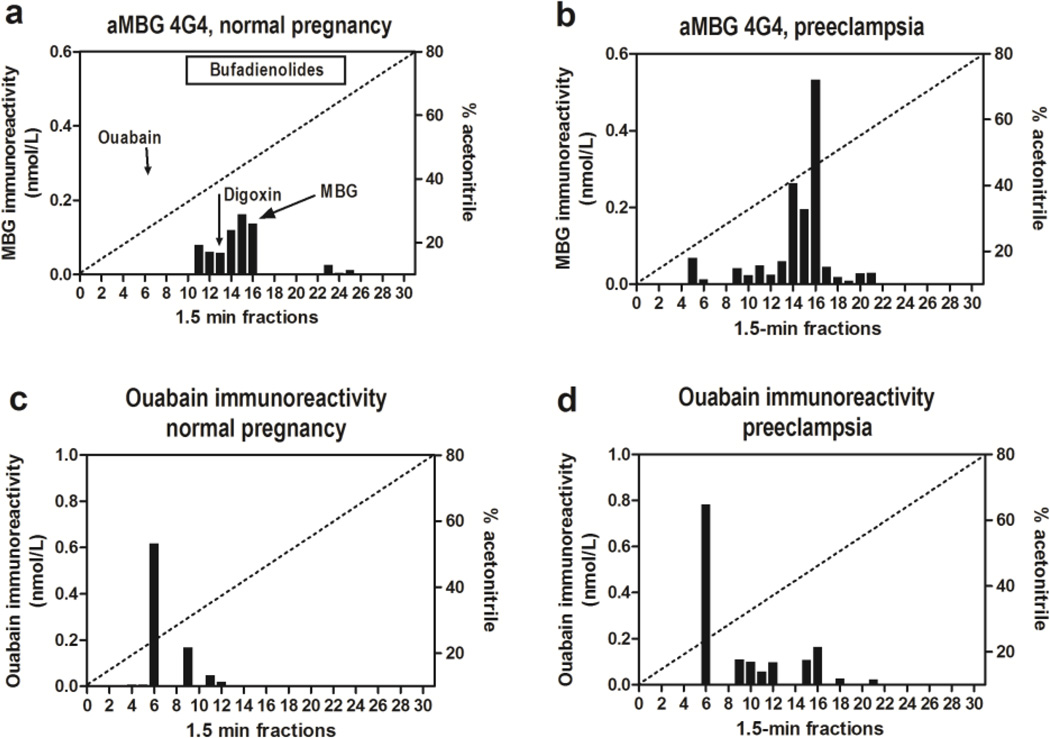
Figure 2.
Fractionation of endogenous CTS from normal and preeclamptic plasma on Zorbax Eclipse XDB-C18 HPLC columns. (a-b) – Pattern of elution of endogenous MBGimmunoreactive material determined by assay based on anti-MBG 4G4 mAb. Retention times of MBG, ouabain and digoxin standards are indicated by arrows. (c-d) - Pattern of elution of endogenous ouabain-immunoreactive material. Bar on the top of panel “a” indicates retention times of bufadienolide standards.