Abstract
Dopamine D2 receptors (D2Rs) play a major role in the function of the prefrontal cortex (PFC), and may contribute to prefrontal dysfunction in conditions such as schizophrenia. Here we report that in mouse PFC, D2Rs are selectively expressed by a subtype of layer V pyramidal neurons that have thick apical tufts, prominent h-current, and subcortical projections. Within this subpopulation, the D2R agonist quinpirole elicits a novel afterdepolarization that generates voltage fluctuations and spiking for hundreds of milliseconds. Surprisingly, this afterdepolarization is masked in quiescent brain slices, but is readily unmasked by physiologic levels of synaptic input which activate NMDA receptors, possibly explaining why this phenomenon has not been reported previously. Notably, we could still elicit this afterdepolarization for some time after the cessation of synaptic stimulation. Besides NMDA receptors, the quinpirole-induced afterdepolarization also depended on L-type Ca2+ channels and was blocked by selective L-type antagonist nimodipine. To confirm that D2Rs can elicit this afterdepolarization by enhancing Ca2+ (and Ca2+-dependent) currents, we measured whole-cell Ca2+ potentials that occur after blocking Na+ and K+ channels, and found quinpirole enhanced these potentials, while the selective D2R antagonist (−)sulpiride had the opposite effect. Thus, D2Rs can elicit a Ca2+-channel dependent afterdepolarization that powerfully modulates activity in specific prefrontal neurons. Through this mechanism, D2Rs might enhance outputs to subcortical structures, contribute to reward related persistent firing, or increase the level of noise in prefrontal circuits.
INTRODUCTION
Dopamine plays a critical role in prefrontal cortex (PFC). First, depleting prefrontal dopamine impairs working memory in monkeys (Brozoski et al., 1979), and genetic variations in prefrontal dopamine catabolism affect both PFC-dependent executive function and prefrontal physiology in humans (Egan et al., 2001). Second, imbalanced prefrontal dopaminergic signaling may contribute to disorders including schizophrenia (Arnsten and Goldman-Rakic, 1998; Winterer and Weinberger, 2004; Kellendonk et al., 2006). Third, reward signals are typically mediated by dopamine (Schultz, 2007), and the past history of reward modulates prefrontal neuron excitability (Bernacchia et al., 2011) and can trigger persistent firing (Histed et al., 2009).
Prefrontal dopamine D2 receptors (D2Rs) play critical roles in cognition. Infusion of D2 agonists and antagonists into PFC modulates working memory and set-shifting in rodents (Druzin et al., 2000; Floresco et al., 2006; St Onge et al., 2011). Systemic administration of D2 agonists in non-human primates affects working memory and elicits “hallucinatory-like” behaviors (Arnsten et al., 1995). In non-human primates, prefrontal D2Rs are specifically necessary for neural activity associated with memory-guided saccades (Wang et al., 2004). Consistent with these animal studies, genetic variation in D2Rs modulates prefrontal activity and working memory in humans (Zhang et al., 2007).
Given that all antipsychotics block D2Rs, and that prefrontal D2Rs play a major role in tasks that are disrupted in schizophrenia, a major hypothesis is that excessive D2R activation contributes to prefrontal dysfunction in schizophrenia (Winterer and Weinberger, 2004; Durstewitz and Seamans, 2008). Prefrontal D2Rs may also contribute to Tourette syndrome and bipolar disorder (Simonic et al., 1998; Minzer et al., 2004; Yoon et al., 2007; Minton et al., 2009; Steeves et al., 2010). Thus, D2Rs play a major role in both normal and pathological prefrontal function. Specifically D2Rs may increase the variability of PFC activity (Winterer and Weinberger, 2004; Durstewitz and Seamans, 2008). Under normal conditions, such variability could facilitate adaptation to a changing environment (Durstewitz et al., 2010; St Onge et al., 2011). However, excessive or imbalanced D2R activation might produce pathological variability that contributes to “prefrontal noise” and cognitive dysfunction in schizophrenia (Winterer and Weinberger, 2004).
We focused on layer V pyramidal neurons in PFC because these neurons contain most prefrontal D2Rs (Lidow et al., 1998; Santana et al., 2009). A few studies have described ways that D2Rs enhance (Wang and Goldman-Rakic, 2004) or suppress (Gulledge and Jaffe, 1998; Tseng and O’Donnell, 2004) excitability in these neurons. A possibly related observation is that dopamine profoundly depolarizes frontal cortex pyramidal neurons in vivo (Bernardi et al., 1982). Nevertheless, specific mechanisms for D2R-modulation of layer V pyramidal neurons in PFC remain elusive.
Here we report two major results about D2Rs in layer V of PFC. First, D2Rs are not uniformly distributed across layer V cell populations, but rather restricted to a specific subpopulation of layer V pyramidal neurons with thick apical tufts, prominent h-current, and subcortical projections. Second, in these neurons, D2Rs elicit a novel afterdepolarization that depends on NMDA receptors and L-type calcium channels, and can drive spiking for hundreds of milliseconds.
METHODS
All experiments were conducted in accordance with procedures established by the Administrative Panels on Laboratory Animal Care at the University of California, San Francisco.
Slice preparation
Slice preparation and intracellular recording followed our published protocol (Sohal and Huguenard, 2005). We cut 250 micron coronal slices from 6–10 week old mice of either sex. Specifically, all electrophysiological experiments showing the quinpirole-induced afterdepolarization were from 9–10 week old mice, except for 4/7 perforated patch experiments, which were from 6–7 week old mice. We used the following mouse lines: wild-type C57Bl/6 mice (Charles River), Drd1::EGFP (line S118; www.gensat.org), Drd1::Cre (line EY262; www.gensat.org), Drd2::EGFP (www.gensat.org), and Drd2::Cre (line ER44; www.gensat.org). We secured the slice by placing a harp along the midline between the two hemispheres.
Intracellular Recording
We obtained somatic whole cell patch recordings from visually identified pyramidal cells in layer V of infralimbic (IL) or prelimbic (PL) cortex using differential contrast videomicroscopy on an upright microscope (BX51WI, Olympus). Recordings were made using a Multiclamp 700A (Axon Instruments). Except when otherwise noted, patch electrodes (tip resistance = 2–6 MOhms) were filled with (in mM): 130 K-gluconate, 10 KCl, 10 Hepes, 10 EGTA, 2 MgCl, 2 MgATP, and 0.3 NaGTP (pH adjusted to 7.3 with KOH). For perforated patch recordings, the pipette solution included 0.02 mg/mL gramicidin D. ACSF contained (in mM): 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl, and 10 glucose. In experiments that used TEA, the amount of NaCl was decreased by a corresponding amount (30 mM) to maintain the osmolarity of the extracellular solution. All recordings were at 32.5 ± 1 °C. Series resistance was usually 10–20MΩ, and experiments were discontinued above30 MΩ.
Injection of virus for ChR2 or EYFP expression
For Cre-dependent expression of ChR2 or EFYP, we used a previously described adeno-associated virus vector that drives Cre-dependent expression of a ChR2-EFYP fusion protein (Sohal et al., 2009). In other cases, we expressed ChR2-EFYP in pyramidal neurons using a previously described adeno-associated virus vector that contains a gene encoding ChR2-EYFP under control of the promoter for CaMKIIα (Yizhar et al., 2011). In each case, we injected 0.5 – 0.75 μL of virus following previously described procedures (Sohal et al., 2009). For experiments in which we recorded from ChR2-negative neurons while stimulating ChR2-positive axons, we injected virus into the contralateral mPFC, and verified that we observed fluorescent soma on the injected side, but not on the contralateral side (which was the location for recording). In 3/5 of these experiments, we drove expression using the Cre-dependent virus in Drd1::Cre mice, while in the other 2/5 experiments we injected the virus carrying the CaMKIIα promoter into wild-type mice. We waited at least 3–4 weeks after virus injection before preparing brain slices. Coordinates for injection into mPFC were (in mm relative to bregma): 1.7AP, 0.3 ML, and −2.75 DV.
Injection of retrogradely transported microspheres for projection targeting experiments
Procedures for injection of these microspheres were similar to those for virus injection. We waited at least 48h after each injection before preparing brain slices. Coordinates for mPFC injections were the same as for virus injections. For injections into MD thalamus, coordinates were (in mm relative to bregma): −1.7AP, 0.3 ML, and −3.5DV. For each experiment, we verified that microspheres were present in the correct target (MD thalamus or mPFC). For injections into MD thalamus we specifically verified that microspheres were not present in nearby structures (e.g. striatum).
Drug application
For electrophysiology, all drugs were dissolved in water (quinpirole, NMDA, DL-AP5, bicuculline methiodide, apamin, and TEA) or DMSO ((−)sulpiride, nimodipine) before being diluted in ACSF, except for and TTX which was dissolved in a pH 4.8 citrate buffer. Throughout the text, “TEA” refers to tetraethylammonium chloride, “AP5” refers to DL-AP5, “bicuculline” refers to bicuculline methiodide, and “sulpiride” refers to (−)sulpiride.
ChR2 stimulation
We stimulated ChR2 in pyramidal neurons using flashes of light generated by a Lambda DG-4 high speed optical switch with a 300W Xenon lamp, and an excitation filter set centered around 470nm, delivered to the slice through a 40x objective (Olympus). Illumination was delivered across a full high-power (40x) field.
Biocytin fills, morphological analysis, and confocal imaging
For experiments in which we studied cell morphology, the intracellular solution contained 0.3% biocytin. Cells filled with biocytin were fixed overnight in a buffered solution containing 4% paraformaldehyde. To visualize filled cells, we washed fixed slices in 0.1 M PBS, then incubated for 40 min in PBS with 1–2% Triton X-100, before incubating for 1 h in PBS with 0.5% Triton and Texas red Avidin D or Fluorescein Avidin D (1:500). Before mounting the slice, we washed it with PBS again. We measured the width of the apical dendritic shaft 5 μm above the soma. To visualize fluorescent cells, we followed a similar protocol omitting incubation with Avidin D. All imaging was carried out on a Zeiss LSM510.
Statistical analysis
We used Student’s t-tests to compare of pairs of groups, unless there were repeated measurements or unpaired observations, in which case we used ANOVA. To compare time constants for 90% decay of the quinpirole-induced afterdepolarization, we first log-transformed these time constants, because their distributions in quinpirole were highly skewed and non-gaussian. Error bars indicate ± 1 S.E.M. unless otherwise specified.
RESULTS
H-current identifies a subpopulation of layer V pyramidal neurons in the PFC that express D2Rs
Initially, we studied whether D2Rs are selectively expressed in previously identified subpopulations of layer V pyramidal neurons. Previous studies have classified layer V pyramidal neurons from somatosensory or frontal cortex based on projection targeting or apical tuft morphology, and found that these characteristics are strongly correlated (Morishima and Kawaguchi, 2006; Hattox and Nelson, 2007). Specifically, layer V pyramidal neurons that project to the thalamus or brainstem have apical dendrites with wider arborizations, thicker shafts, and a greater number of primary branches, than do apical dendrites originating from layer V pyramidal neurons that project to contralateral cortex and/or striatum. This suggests that layer V pyramidal neurons in neocortex can be divided into at least two subpopulations, “thick-tufted” neurons that project to thalamus or brainstem, and “thin-tufted” neurons that project to striatum or contralateral cortex. Notably, excitatory and inhibitory connectivity differs between these subpopulations (Wang et al., 2006; Otsuka and Kawaguchi, 2008; Brown and Hestrin, 2009). A recent study found that in prefrontal layer V pyramidal neurons that project to contralateral cortex (CC) or brainstem, levels of the hyperpolarization-activated cyclic nucleotide gated cation h-current (Ih) are low or high, respectively (Dembrow et al., 2010). An analogous relationship holds in motor cortex (Sheets et al., 2011).
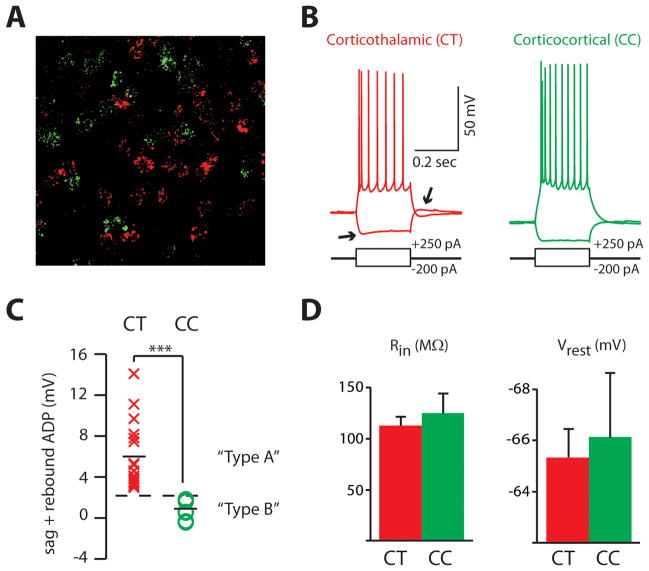
Given these previous findings, we first sought to confirm that we could use h-current to classify neurons that have different projection targeting. If prefrontal corticothalamic (CT) and corticopontine neurons have similar properties, since they are both thick-tufted, then the level of h-current in CT neurons should be greater than that in CC neurons. Indeed, we found that h-current could be used to reliably distinguish CT and CC neurons in layer V of medial PFC (Fig. 1A–D). To identify CT or CC neurons, we injected retrogradely transported fluorescently labeled latex microspheres (Retrobeads; Lumafluor) into the ipsilateral medial dorsal thalamus or the contralateral PFC. As illustrated in Fig. 1A, CT and CC neurons were distinct populations. We made whole cell patch-clamp recordings from identified CT or CC neurons in layer V of the medial PFC (mPFC). Although CT and CC neurons had similar resting membrane potentials and input resistances (Fig. 1D), they had significantly different levels of h-current (measured as the sum of the voltage sag and rebound depolarization in response to a hyperpolarizing current pulse) (Fig. 1B,C; p < 0.01; n = 18 and 8 CT and CC neurons, respectively). In fact, the distributions of h-current from CT and CC neurons were completely nonoverlapping (Fig. 1C).
FIGURE 1. H-current distinguishes two populations of layer V pyramidal neurons that differ in their projection targets.
(A) High power confocal image of layer V of mPFC showing the distribution of fluorescently labeled retrogradely transported microspheres within individual neurons. Microspheres were injected into MD thalamus (red) or the contralateral PFC (green). Bar = 10 μm. (B) Sample recordings from corticothalamic (CT) or corticocortical (CC) pyramidal neurons identified using retogradely transported microspheres showing responses to hyperpolarizing or depolarizing current injection. Note the voltage sag and rebound afterdepolarization in response to hyperpolarizing current injection that is visible in the CT neuron but not the CC neuron (arrows). (C) The amount of h-current, measured as the sum of the voltage sag and rebound afterdepolarization in response to hyperpolarizing current pulses, in CT (n = 18) and CC neurons (n = 8). Thick horizontal bars indicate the means of each distribution, and the dotted line indicates a threshold that unambiguously separates the two nonoverlapping distributions. (D) Input resistances (Rin) and resting membrane potentials (Vrest) for CT and CC neurons. ***p < 0.001.
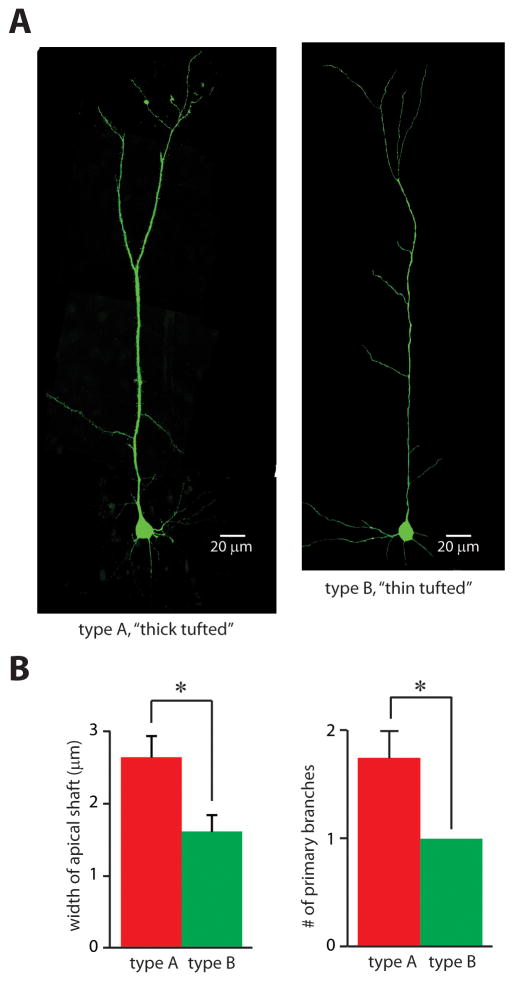
Thus, we could define a threshold level of h-current that would unambiguously separate CT and CC neurons. We refer to layer V pyramidal neurons above this threshold as “type A” neurons, and those below this threshold as “type B neurons.” Based on the studies outlined above, we would predict that type A neurons (more h-current) would be thick-tufted whereas type B neurons (minimal h-current) should be thin-tufted. Indeed, after filling layer V pyramidal neurons in mPFC with biocytin and visualizing them via confocal microscopy (Fig. 2A), we found that type A neurons (n = 4) had a greater number of primary branches (p < 0.05) and wider apical shafts (p < 0.05) than type B neurons (n = 4) (Fig. 2B). Thus, consistent with previous studies, the level of h-current differentiates two subpopulations of layer V pyramidal neurons that project to different targets and have different apical tuft morphologies.
FIGURE 2. Type A and B pyramidal neurons have different morphologies.
(A) Confocal images of representative neurons in which the amount of h-current falls either above (“type A,” left image) or below (“type B,” right image) the threshold in Fig. 1C. (B) Type A and B neurons differ in the widths of the shafts of their apical dendrites (left) and in the number of primary branches of their apical dendrites (right) (n = 4 neurons in each group). *p < 0.05.
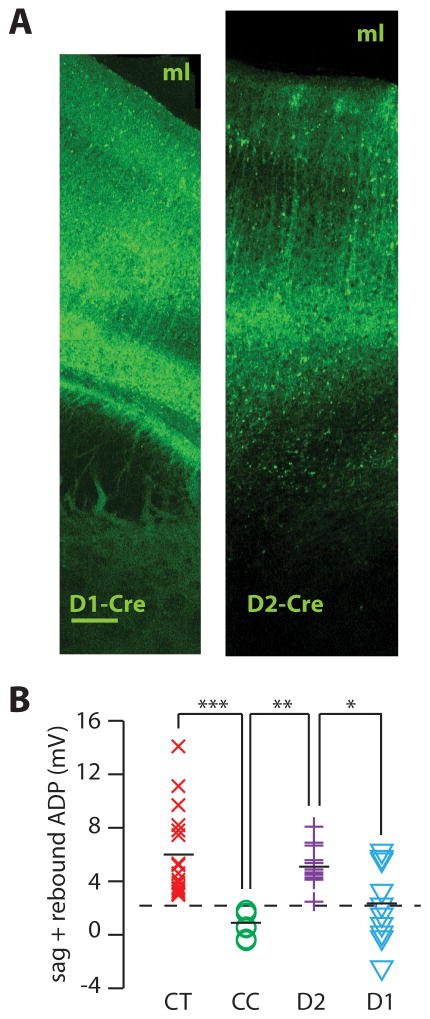
Next, we asked whether D2Rs are selectively expressed within these two subpopulations of layer V pyramidal neurons in the PFC. To answer this question, we recorded from visually identified neurons expressing fluorescent proteins under control of promoters for D1Rs or D2Rs (Fig. 3A). Specifically, we recorded from fluorescent neurons in Drd1::EGFP transgenic mice (n = 4) (line S118), Drd1::Cre transgenic mice (line EY262) injected with virus containing a Cre-dependent construct for ChR2-EYFP (Sohal et al., 2009) (n = 6), Drd2::EGFP transgenic mice (n = 7), or Drd2::Cre transgenic mice (line ER44) injected with adeno-associated virus (AAV) containing a Cre-dependent construct for ChR2-YFP (n = 7). These transgenic mice have been widely used to study D1R and D2R-expressing neurons in the striatum (Gong et al., 2003; Lobo et al., 2006; Kravitz et al., 2010), and this AAV drives EYFP expression that is highly specific for Cre-expressing neurons (Sohal et al., 2009). We found that all of the presumed D2R-expressing neurons (fluorescent neurons from Drd2::EGFP or Drd2::Cre mice) were “type A”, i.e. had a level of h-current current above the threshold that separates CT and CC neurons, whereas presumed D1R-expressing neurons included both type A and type B neurons (Fig. 3B). Of course, other subpopulations of layer V pyramidal neurons might also express D2Rs, but not be labeled by either transgenic line we used. We identified D2R-expressing neurons using two distinct transgenic lines in order to minimize this possibility. The following experiments provide additional confirmation for our finding that D2R expression is restricted to a specific subpopulation of layer V neurons, by showing that activating D2Rs elicits a novel cellular effect that is also restricted to this same subpopulation.
FIGURE 3. D2Rs are selectively expressed in Type A pyramidal neurons which can be distinguished using the h-current.
(A) Low-power confocal images of infralimbic cortex showing the pattern of fluorescence in Drd1-Cre (D1-Cre) and Drd2-Cre (D2-Cre) transgenic mice injected with virus to drive Cre-dependent expression of ChR2-YFP. The corpus callosum and midline lie below and above both images, respectively. ml, midline. Bar = 0.1 mm (both images are to the same scale). (B) The amount of h-current (measured as above) in identified corticothalamic (CT, n = 18), corticocortical (CC, n = 8), D2R expressing (D2, n = 14), or D1R expressing (D1, n = 10) pyramidal neurons in layer V of mPFC. The dotted line indicates the threshold that separates the distributions of h-current from CT and CC neurons. * = p < 0.05, ** = p < 0.01.
Synaptic activity unmasks a quinpirole-induced afterdepolarization in Type A neurons
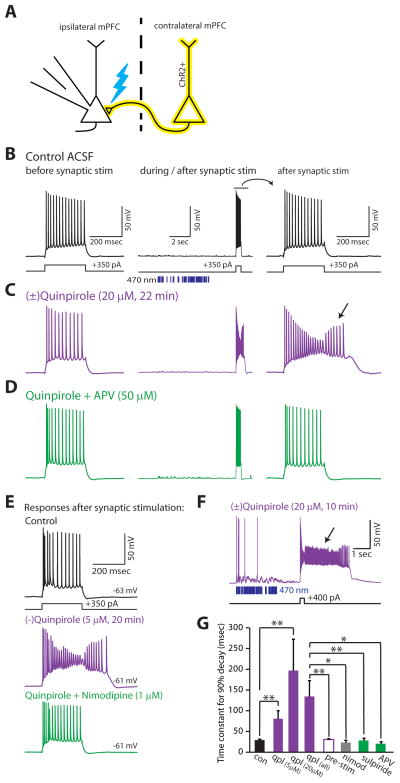
We measured effects of D2R activation in type A and B neurons, and surprisingly, at baseline, the D2R agonist quinpirole had minimal effects on the responses of type A neurons to depolarizing current pulses (300–400 pA, 250–500 msec; left panels of Fig. 4B–D). However, when these current pulses were preceded by optogenetic stimulation of excitatory synapses in layer V, quinpirole dramatically altered the responses of these neurons to depolarizing current. Specifically, under these conditions, quinpirole (5–20 μM) elicited a prominent afterdepolarization in 12/12 type A neurons (middle and right panels of Fig. 4C; Fig. 4E,F). This afterdepolarization could generate spikes and extend for up to several seconds after the end of the current pulse (Fig. 4F). In addition to this afterdepolarization, quinpirole caused a progressive decrease in spike height during the current pulse (Fig. 4C–F). Despite eliciting this dramatic afterdepolarization, both 5 μM (−)quinpirole and 20 μM (±)quinpirole had minimal effects on the resting membrane potential (5 μM: control Vrest = −61.7 ± 1.4 mV, quinpirole Vrest = −60.8 ± 1.5 mV; p = 0.32, n = 8; 20 μM: control Vrest = −62.3 ± 1.8 mV, quinpirole Vrest = −60.9 ± 1.5 mV; p = 0.42, n = 5). For these experiments, we expressed ChR2 in pyramidal neurons in the contralateral mPFC using viral injection (Fig. 4A; Methods). We then recorded from type A neurons that did not express ChR2 while stimulating ChR2-positive corticocortical fibers with trains of randomly occurring light flashes (470 nm; 2.5 msec duration; intensity: ~2 mW; rate: 5–50 Hz; train duration: 2.5 sec; 5 trains were delivered with an intertrain interval of 13 sec). As illustrated in Fig. 4, this led to relatively weak synaptic input that usually evoked zero or only a few spikes in the postsynaptic neuron.
FIGURE 4. Synaptic stimulation unmasks a novel D2R-mediated afterdepolarization in specific layer V pyramidal neurons.
(A) Experimental design: We recorded from ChR2 negative layer V neurons while stimulating ChR2 positive axons from the contralateral mPFC with trains of light flashes (470 nm, 2.5 msec, ~2 mW). (B) Responses of a type A layer V pyramidal neuron to current injection before (left panel) and immediately following (middle and right panels) optogenetic stimulation of synaptic inputs. Blue bars indicate the times of light flashes. (C) Prior to synaptic stimulation, no quinpirole-induced afterdepolarization is observed, however the same current injection elicits a marked afterdepolarization (along with spike height rundown) following weak synaptic stimulation. (D) The quinpirole-induced afterdepolarization is eliminated by the addition of AP5. (E) Lower doses of quinpirole (5 μM) also induce an afterdepolarization following synaptic stimulation, which can be blocked by nimodipine (1 μM). (F) Recording from a type A neuron showing a prolonged quinpirole-induced afterdepolarization following synaptic stimulation. (G) Average time constants for the membrane potential to return to baseline following a depolarizing current pulse (300–400 pA, 250–500 msec) delivered immediately following the pattern of synaptic stimulation shown above. Data is shown for control conditions (black; n=12), quinpirole (purple; 5 μM, n=7; 20 μM, n=6), quinpirole in the absence of synaptic stimulation (hollow purple bar; 5 μM, n=6), nimodipine (gray; 1 μM, n=3), sulpiride (green; 5 μM, n=4), or AP5 (green; 50 μM, n=3). * = p < 0.05, ** = p < 0.01.
We quantified the quinpirole-induced afterdepolarization by measuring the time for the membrane potential to decay back to within 90% of the baseline membrane potential following a depolarizing current pulse (Fig. 4G). To confirm that D2Rs mediate the quinpirole-induced afterdepolarization, we used the selective D2R antagonist (−)sulpiride. Many studies have used sulpiride at doses up to 10 μM to confirm that various phenomena are mediated by D2Rs (Kreitzer and Malenka, 2005; Ramanathan et al., 2008; Ding et al., 2010). Indeed, we found that 5 μM (−)sulpiride eliminated the quinpirole-induced afterdepolarization in 4/4 cells (Fig. 4G). This dose is ~10-fold less than the Ki for (−)sulpiride binding to D1Rs in expression systems (Seeman and Van Tol, 1994), and should thus be highly selective for D2Rs in brain slices. The quinpirole-induced depolarization could be reversed in other ways as well: by addition of the NMDA-R antagonist AP5 (50 μM; n = 3/3 cells; Fig. 4C,G); by addition of the selective L-type Ca2+ channel antagonist nimodipine (1 μM; n= 3/3 cells; Fig. 4C,G). Furthermore, as shown in Fig. 4G, the duration of the quinpirole-induced afterdepolarization was larger for 20 μM (±)quinpirole (n=6) than for 5μM (−)quinpirole (n=7), although this difference did not reach statistical significance.
Many studies have activated D2Rs using 10 μM quinpirole (Wang and Goldman-Rakic, 2004; Kreitzer and Malenka, 2005; Ramanathan et al., 2008; Sidiropoulou et al., 2009), similar to the doses we have used (5 or 20 μM). Nevertheless, these doses are higher than those used in other studies of PFC, e.g. 1–2 μM (Tseng and O’Donnell, 2007; Tseng et al., 2008). We will address possible reasons for these discrepancies in the Discussion, although we remain confident that D2Rs are required for the quinpirole-induced afterdepolarization because this phenomenon (1) can be elicited by moderate doses of quinpirole (5 μM), (2) occurs selectively in D2R-expressing neurons, and (3) is blocked by the specific D2R antagonist sulpiride (5 μM). Of course, we cannot rule out the possibility that in addition to D2Rs, other receptors also play a role. Since we obtained the most prominent afterdepolarization using 20 μM (±)quinpirole, we used this dose for subsequent experiments. As described below, all of the effects we observed using 20 μM (±)quinpirole were reversed by (−)sulpiride (5 μM), confirming that they require D2Rs.
Activating NMDA receptors also unmasks the quinpirole-induced afterdepolarization
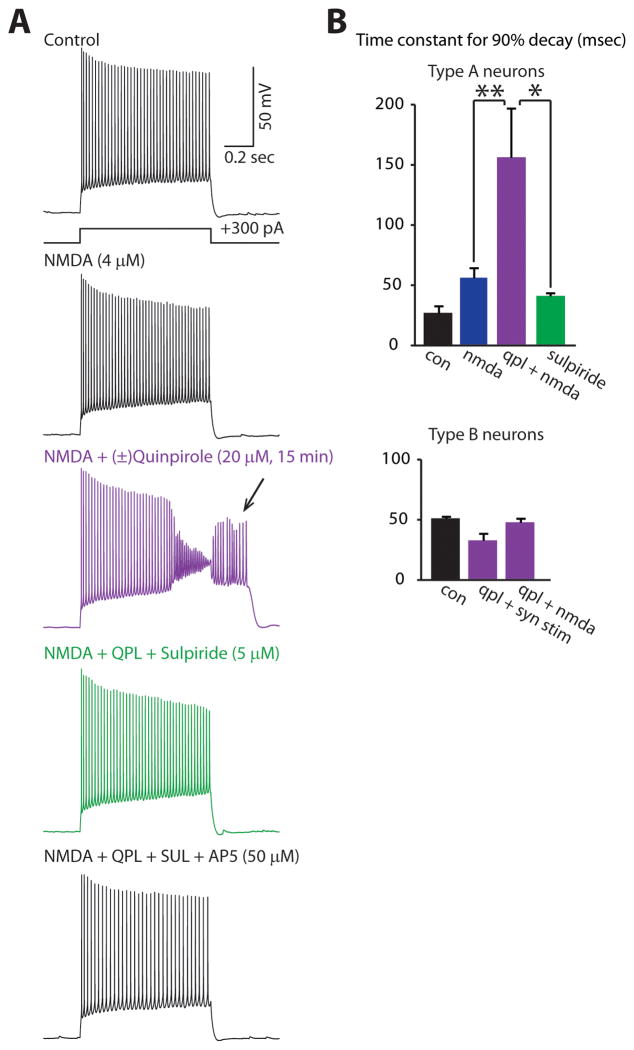
Given that AP5 blocks the ability of synaptic stimulation to unmask the quinpirole-induced afterdepolarization, we tested whether modest levels of NMDA receptor activation might suffice to unmask this effect. Indeed, we observed the quinpirole-induced afterdepolarization when we included a low concentration of NMDA (4 μM) in the bath (Fig. 5A,B; n=4). Notably, the afterdepolarization was not induced by this concentration of NMDA alone, and was reversed by (−)sulpiride (5 μM; Fig. 5A,B; n=4). Fig. 5B quantifies and summarizes these effects. The concentration of NMDA we used is within the range used by previous studies to elicit network activity in prefrontal brain slices (3–8 μM) (Tseng and O’Donnell, 2005; Stewart and Plenz, 2006).
FIGURE 5. NMDA can unmask the quinpirole-induced afterdepolarization in type A neurons.
(A) Responses of a type A neuron to depolarizing current pulses in various pharmacologic conditions showing that bath application of quinpirole and NMDA, but not NMDA alone, induces an afterdepolarization (arrow) that is reversed by sulpiride. (B, top) Summary data showing the effect of quinpirole, NMDA, and sulpiride on the time constant for the membrane potential to return to baseline following depolarizing current pulses (350 pA, 250 msec) in type A neurons (n=4 for each condition): control (black), NMDA (4 μM, blue), (±)quinpirole (20 μM) + NMDA (purple), or sulpiride (5 μM) + quinpirole + NMDA (green). (B, bottom) Quinpirole does not elicit a similar afterdepolarization in type B neurons. The time constant for the membrane potential to return to baseline following depolarization current pulses (350 pA, 250 msec) in Type B neurons is shown for various conditions: control (black, n=3), (±)quinpirole (20 μM) following optogenetic synaptic stimulation (n=3; purple), (±)quinpirole (20 μM) plus NMDA (4 μM) (n=4; purple). *p < 0.05, **p < 0.01.
The quinpirole-induced afterdepolarization is absent from Type B neurons
We also applied (±)quinpirole (20 μM) to type B neurons (n=7), and measured their responses to depolarizing current pulses (350 pA, 250 msec). As in the experiments with type A neurons, depolarizing current pulses either occurred immediately following optogenetic stimulation of excitatory synapses in layer V (n=3), or with 4 μM NMDA in the bath (n=4). None of these experiments elicited a quinpirole-induced afterdepolarization in type B neurons. This is quantified in Fig. 5B, which shows how various conditions affect the time for the membrane potential to return to baseline following a depolarizing current pulse in type B neurons.
The quinpirole-induced afterdepolarization occurs in perforated-patch recordings
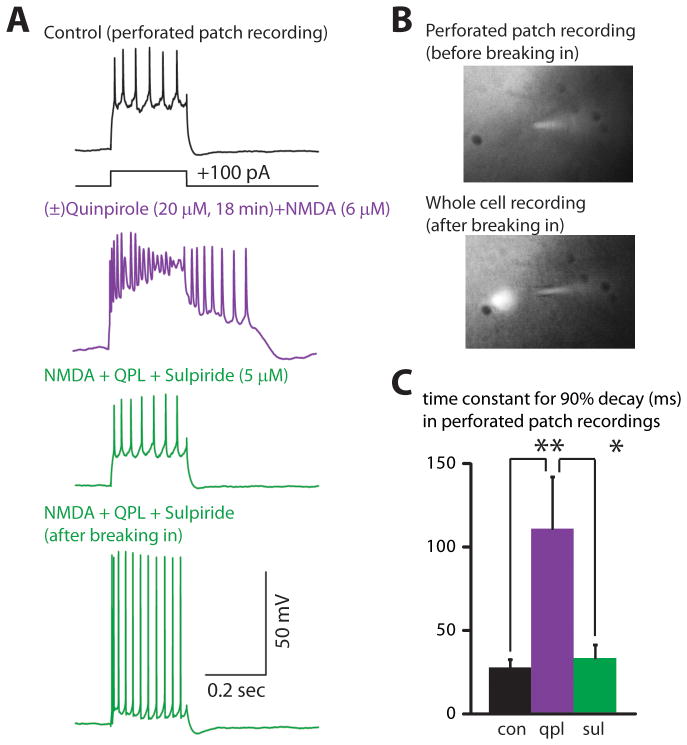
To rule out the possibility that the afterdepolarization only occurs after dialyzing neurons with our intracellular solution, we verified that we could observe this effect during gramicidin perforated-patch recordings (Methods). Indeed, during perforated-patch recordings from type A neurons, co-application of (±)quinpirole (20 μM) and NMDA (6 μM) elicited an afterdepolarization that was reversed by adding the D2R antagonist (−)sulpiride (5 μM) (Fig. 6A,C; n=5). Two additional details of this experiment were notable. First, in this experiment, we identified type A neurons by injecting retrobeads into MD thalamus to label CT neurons. 5/5 labeled CT neurons exhibited the quinpirole-induced afterdepolarization, providing additional evidence that CT neurons express D2Rs. Second, although we monitored the bridge balance for sudden changes indicative of a shift from a perforated-patch to whole cell recording (compare top 3 vs. bottom panels of Fig. 6A), in some cases we also included fluorescent dye in the pipette (0.05% Lucifer Yellow; n=2). As shown in Fig. 6B, this fluorescent dye was excluded from the neuron while in the perforated-patch configuration (top image), but entered the neuron after breaking in and shifting to a whole cell configuration (bottom image).
FIGURE 6. Quinpirole also induces an afterdepolarization during perforated-patch recordings from type A neurons.
(A) Recordings from a type A neuron in perforated-patch configuration (top three panels) showing the quinpirole-induced afterdepolarization that occurs in the presence of NMDA, and is reversed by sulpiride. The bottom panel shows a recording from the same neuron after breaking in and shifting to a whole cell recording. (B) Fluorescent dye in the recording pipette was excluded from the neuron while in the perforated-patch configuration (top image), but entered the neuron after breaking in and shifting to a whole cell configuration (bottom image). (C) Summary data showing that (±)quinpirole (20 μM) plus NMDA (6 μM) prolongs the time constant for the membrane potential to return to baseline following depolarizing current pulses (350 pA, 250 msec), and that this is reversed by the addition of sulpiride (5 μM) (n=5). * = p < 0.05, ** = p < 0.01.
Quinpirole prolongs Ca2+-mediated plateau potentials
We next sought to characterize the ion channels that contribute to the quinpirole-induced afterdepolarization. Similar afterdepolarizations have been observed in response to 5-HT in turtle motoneurons (Hounsgaard and Kiehn, 1989), in response to D1R stimulation and/or synaptic stimulation in striatal projection neurons (Hernandez-Lopez et al., 1997; Vergara et al., 2003), in frog olfactory bulb neurons (Hall and Delaney, 2002), and in nigral GABAergic neurons (Lee and Tepper, 2007). Notably, these other afterdepolarizations also caused spike height rundown very similar to what we observed with quinpirole (e.g. Fig. 4–6). Each of these other afterdepolarizations were generated by combinations of Ca2+ influx via L-type Ca2+ channels and/or NMDA receptors (NMDA-Rs), and the Ca2+-activated nonselective cationic conductance (ICAN). Our experiments suggest that a similar mechanism mediates the quinpirole-induced afterdepolarization, since it can be eliminated by antagonizing either L-type Ca2+ channels or NMDA-Rs.
An ideal experiment would be to measure how quinpirole affects L-type Ca2+ currents. However, none of these previous studies of similar afterdepolarizations measured L-type Ca2+ currents directly (Hounsgaard and Kiehn, 1989; Hernandez-Lopez et al., 1997; Hall and Delaney, 2002; Vergara et al., 2003; Lee and Tepper, 2007), because poor space clamp make it notoriously difficult to isolate and measure regenerative voltage-dependent currents in intact pyramidal neurons. In fact, attempting to do so can lead to spurious results (Maurice et al., 2001). Indeed, we attempted to directly measure the effects of quinpirole on voltage-dependent Ca2+ currents, but these recordings suffered from poor space clamp. Similarly, it is extremely challenging to directly measure Ca2+-dependent currents such as ICAN, because the intracellular Ca2+ concentration varies during current clamp or voltage clamp recordings. Because of these issues, numerous studies indirectly measured these Ca2+ and Ca2+-dependent currents by studying plateau potentials which occur after blocking voltage-dependent Na+ and/or K+ currents (Forscher and Oxford, 1985; Hounsgaard and Kiehn, 1989; Hernandez-Lopez et al., 1997; Young and Yang, 2004; Lee and Tepper, 2007).
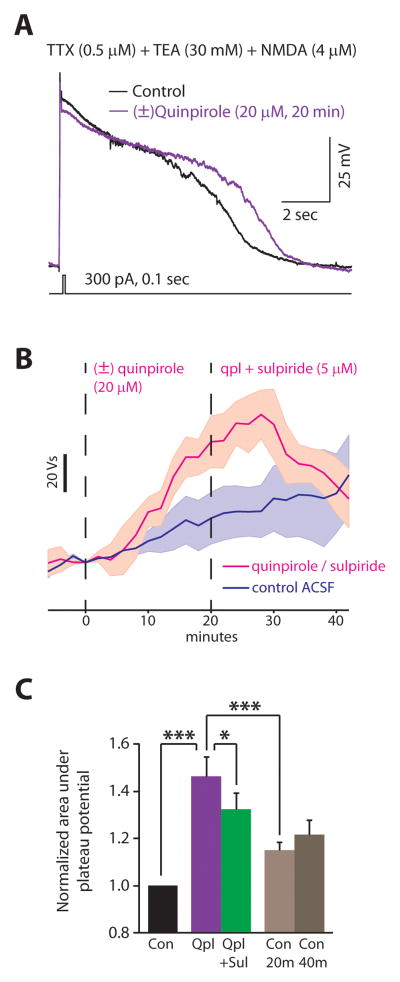
We followed this well-established approach and confirmed that quinpirole enhances Ca2+ and/or Ca2+-dependent currents that produce the quinpirole-induced afterdepolarization, by recordeding plateau potentials that occur after application of TTX (0.5 μM) and TEA (30 mM) to block Na+ and K+ currents (Fig. 7A; Methods). In these experiments, a brief, strong depolarizing current pulse (300 pA, 100 msec) triggers a high-threshold Ca2+ spike that is followed by a long plateau potential. These plateau potentials last several seconds. As a result, although they may contribute to the initial Ca2+ spike, T-type Ca2+ channels will be inactivated during the plateau potential. Furthermore, a previous study of layer V-VI pyramidal neurons in PFC found that under these conditions, evoked Ca2+ spikes are driven primarily by L-type Ca2+ channels (Young and Yang, 2004). Based on our earlier experiments, we recorded these plateau potentials in the presence of 4 μM NMDA. Thus, these plateau potentials are driven primarily by a combination of L-type Ca2+ currents, NMDA-R mediated currents, and Ca2+-dependent currents such as the Ca2+-activated nonselective cationic current, ICAN. Other slowly or non-inactivating high-threshold Ca2+ currents, e.g. N-type, may also contribute, but likely play a much smaller role (Young and Yang, 2004). We observed a clear increase in these plateau potentials after applying (±)quinpirole (20 μM) for 20 min (Fig. 7B,C). This effect was partially reversed by adding (−)sulpiride (5 μM) for 20 min (Fig. 7B,C; n = 4 cells; p < 0.001 control vs. quinpirole, p < 0.05 quinpirole vs. quinpirole + sulpiride by repeated measures ANOVA using cell and condition as factors and after correcting for multiple comparisons). There was usually a slight increase in the plateau potential over time, so we also measured the size of the plateau potential in control ACSF after 20 min (corresponding to the time of quinpirole application) and 40 min (corresponding to the time of sulpiride application). The increase in the size of the plateau potential was much larger in cells for which we applied quinpirole (n = 4), compared to cells maintained in control ACSF (n = 4) (p < 0.001 by ANOVA using cell and condition as factors and after correcting for multiple comparisons). Thus, although it was not possible to directly measure L-type Ca2+ currents or Ca2+-dependent currents, we did confirm that quinpirole enhances Ca2+ and/or Ca2+-dependent currents that produce plateau potentials when Na+ and K+ channels are blocked. This, together with our experiments using nimodipine, NMDA, and AP5, strongly suggest that like other similar afterdepolarizations, the quinpirole-induced depolarization is mediated by a combination of Ca2+ currents mediated by L-type channels and NMDA-Rs, as well as depolarizing Ca2+-dependent currents, e.g. ICAN.
FIGURE 7. Quinpirole reversibly prolongs calcium-dependent plateau potentials.
(A) A short current pulse elicits a brief Ca2+ spike that is followed by a prolonged plateau potential in a type A neurons after application of TTX and TEA. Each experiment recorded plateau potentials in control conditions, then while applying (±)quinpirole (20 μM), and finally while applying quinpirole + sulpiride (5 μM). (B) We quantified the size of plateau potentials by measuring the area under the voltage trace. The average size of the plateau potentials are shown as a function of time (n=4 cells in each condition). For the magenta trace, t=0 represents the beginning of quinpirole application, and quinpirole and sulpiride were both applied after t=20 min. The dark blue trace represents recordings in control ACSF. Shaded regions represent ± 1 S.E.M. (C) Summary data for the size of plateau potentials in each condition. Each bar represents data collected from 5 min before until 5 min after the end of drug application, or corresponding timepoints during recordings in control ACSF. We measured plateau potentials every 5 min during this period and used repeated measures ANOVA and corrections for multiple comparisons to assess statistical significance. * = p < 0.05, *** = p < 0.001.
Intracellular BAPTA eliminates the quinpirole-induced afterdepolarization
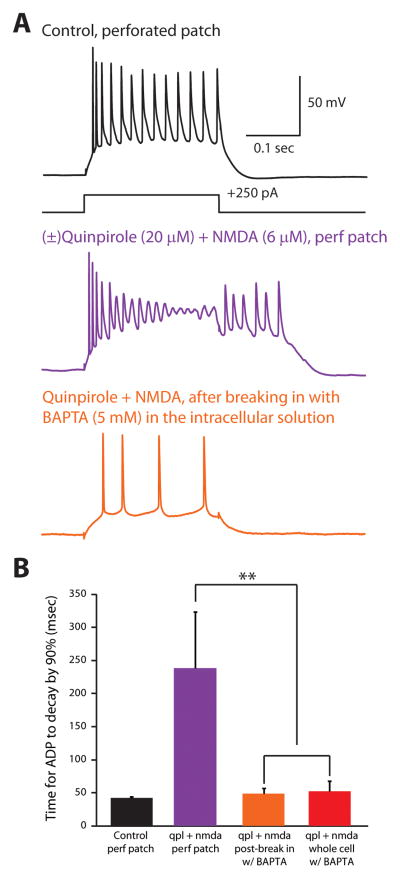
The experiments described above leave open whether the quinpirole-induced afterdepolarization results simply from Ca2+ currents themselves, or whether the activation of Ca2+-dependent currents, e.g. ICAN, is also required. To provide additional evidence that Ca2+ influx is specifically required for the quinpirole-induced afterdepolarization, we tested whether the afterdepolarization is blocked by the Ca2+ chelator BAPTA, which blocks or attenuates downstream effects of Ca2+ but not Ca2+ currents themselves. We made perforated patch recordings and included BAPTA (5 mM) in the intracellular solution. As in previous experiments, we made perforated patch recordings from type A neurons, and observed an afterdepolarization after applying quinpirole and NMDA (Fig. 8A). However, immediately after breaking in and switching to a whole-cell recording configuration, the afterdepolarization and other effects of quinpirole (e.g. decreasing spike heights) disappeared (Fig 8A,B; n = 3/3 cells).
FIGURE 8. The Ca2+ chelator BAPTA eliminates the quinpirole-induced afterdepolarization.
(A) Perforated patch recording from a type A pyramidal neuron in control conditions (top) and after application of quinpirole + NMDA elicits an afterdepolarization (middle). Bottom panel: in the same neuron, after breaking in and switching to a whole cell recording configuration, the quinpirole-induced afterdepolarization is abolished. BAPTA (5 mM) is present in the pipette solution. (B) Summary data showing time constants for the membrane potential to return to baseline following depolarizing current pulses (50–150 pA, 250 msec) under various conditions. “Control” = perforated patch recordings in control ACSF (black; n = 3), “qpl + NMDA, perf patch” = the same perforated patch recordings after applying quinpirole and NMDA (orange; n = 3), “qpl + NMDA, post-break in w/BAPTA” = whole cell recordings from the same cells that were initially recorded in perforated patch configuration (in quinpirole and NMDA) (purple; n = 3), “qpl + NMDA, whole cell w/BAPTA” = recordings from cells that broke in and switched to whole cell configuration during the application of quinpirole + NMDA (red; n = 5). ** = p < 0.01.
In 5 other neurons, we broke in and switched to a whole-cell configuration (with BAPTA in the pipette) while applying quinpirole. In all of these cases, BAPTA prevented quinpirole from inducing an afterdepolarization (Fig. 8B). Note that the intracellular solution we used in previous experiments contained another Ca2+ chelator – EGTA. However, it is known that BAPTA but not EGTA prevents Ca2+ from activating ICAN (Forscher and Oxford, 1985; Hall and Delaney, 2002). In particular, the fact that BAPTA, but not EGTA, blocks the quinpirole-induced afterdepolarization suggests that this phenomenon depends on ICAN (which is blocked by BAPTA but not EGTA) but not intracellular Ca2+ acting as a second messenger (which would be blocked equally well by EGTA and BAPTA) (Forscher and Oxford, 1985).
Blocking SK channels can also unmask a quinpirole-induced afterdepolarization
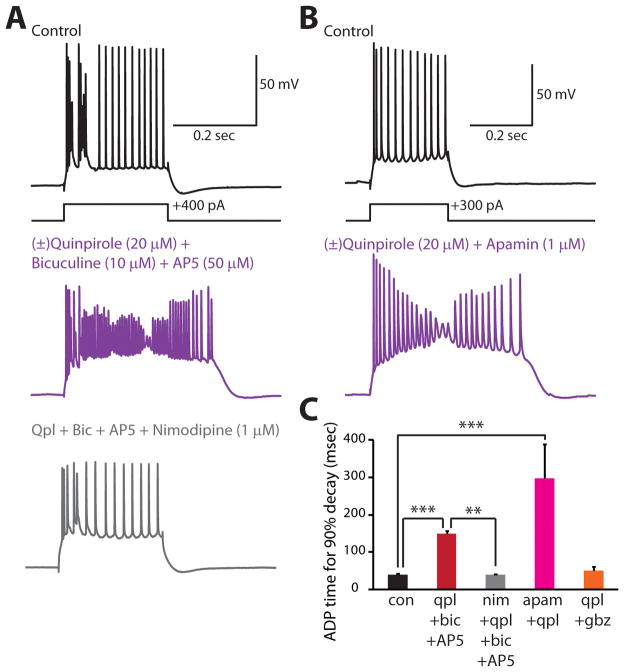
A previous study of thick-tufted layer V pyramidal neurons in the mPFC (Wang and Goldman-Rakic, 2004) found that D2R activation reduces the threshold for bursts evoked by synaptic stimulation in the presence of bicuculline and AP5. This study focused on synaptically evoked bursts lasting ~ 50 msec instead of afterdepolarizations lasting for hundreds of msec or seconds, and the results of that study were obtained under conditions of GABAA and NMDA receptor blockade. Moreover, that study left open whether D2R-mediated increases in synaptically-evoked bursting result from pre- or post-synaptic effects. Nevertheless, that study, like ours, suggests that D2Rs can enhance the excitability of thick-tufted layer V pyramidal neurons in the mPFC. Thus, the increased bursting observed in that study may result from the same mechanisms which we have found produce the quinpirole-induced afterdepolarization. If this is true, application of bicuculline and AP5 may suffice to unmask the quinpirole-induced afterdepolarization, even in the absence of synaptic stimulation. Indeed, after applying (±)quinpirole (20 μM), bicuculline (10 μM), and AP5 (50 μM), we observed an afterdepolarization in type A neurons very similar to the quinpirole-induced afterdepolarization seen in previous experiments (Fig. 9A,C; n = 3/3 neurons). Moreover, this afterdepolarization was blocked by nimodipine (1 mM; n = 3 cells). Thus, even when NMDA-Rs are blocked, quinpirole can still elicit an afterdepolarization, and this afterdepolarization depends on L-type Ca2+ channels. This suggests that L-type Ca2+ channels play a major role in quinpirole-induced afterdepolarizations, whereas NMDA-Rs may facilitate this afterdepolarization, but are not required under all conditions.
FIGURE 9. Blocking SK channels and applying quinpirole produces an afterdepolarization that requires L-type Ca2+ channels.
(A) Responses of a type A neuron to depolarizing current pulses in various pharmacologic conditions showing that bath application of quinpirole, bicuculine, and AP5 induces an afterdepolarization (middle panel of A) that is reversed by nimodipine (bottom panel of A). (B) Responses of another type A neuron showing that application of quinpirole and apamin induces a similar afterdepolarization (middle panel of B). (C) Summary data showing the effect of various conditions on the time constant for the membrane potential to return to baseline following depolarizing current pulses (350 pA, 250 msec) in type A neurons: control (black, n = 10), quinpirole + bicuculline + AP5 (red, n = 3), quinpirole + bicuculline + AP5 + nimodipine (gray, n = 3), apamin + quinpirole (magenta, n = 3), quinpirole + gabazine (10 μM, orange, n = 4). **p < 0.01, ***p < 0.001.
In addition to blocking GABAA receptors, bicuculline also blocks SK-type Ca2+ dependent K+ channels (Johnson and Seutin, 1997; Debarbieux et al., 1998). Therefore, we tested whether the afterdepolarization observed in type A neurons after applying quinpirole, bicuculline, and AP5, depends on the blockade of GABAA receptors and/or SK channels. We found that co-application of quinpirole and the GABAA antagonist gabazine (10 μM) did not elicit an afterdepolarization (Fig. 9C; n = 3 cells), whereas co-application of quinpirole and the selective SK channel antagonist apamin (10 μM) did elicit an afterdepolarization in type A neurons (Fig. 9B,C; n = 3 cells). These results suggest that (1) the mechanism of the quinpirole-induced afterdepolarization may be relevant to the effects of D2Rs on synaptically evoked bursting observed in a previous study, (2) SK channel blockade can unmask quinpirole-induced afterdepolarizations in the absence of synaptic stimulation, and (3) like the quinpirole-induced afterdepolarization which occurs in the presence of synaptic stimulation, the mechanism through which quinpirole increases type A neuron excitability when bicuculline is present also depends on L-type Ca2+ channels.
DISCUSSION
We have characterized a novel afterdepolarization elicited by D2Rs in the mPFC. This afterdepolarization depends on L-type Ca2+ channels and NMDA-Rs. The afterdepolarization and D2R expression both occur within a specific subpopulation of layer V pyramidal neurons that have a characteristic morphology (thick-tufted), projection targets (thalamus), and electrophysiological signature (prominent h-current). This suggests that as in striatum, prefrontal D2Rs are restricted to specific cell populations.
As described below, the quinpirole-induced afterdepolarization may underlie numerous functional and pathological effects of prefrontal D2Rs including enhancing firing during memory-guided saccades (Wang et al., 2004), generating persistent reward-related firing (Histed et al., 2009; Bernacchia et al., 2011), or increasing the variability of activity (Winterer and Weinberger, 2004; Durstewitz and Seamans, 2008). In addition, this afterdepolarization may alter prefrontal output to structures including the mediodorsal thalamus, which is important for processes such as corollary discharge (Sommer and Wurtz, 2006). Notably, the quinpirole-induced afterdepolarization may represent a mechanism for the long-standing observation that dopamine application to frontal cortical neurons in vivo produces a depolarization accompanied by reduced spike heights (Bernardi et al., 1982). Another study also observed reduced spike heights following D2R activation in deep layer mPFC neurons (Sesack and Bunney, 1989). From an extracellular perspective, reduced spike heights would look like an inhibition in firing. Thus, the quinpirole-induced afterdepolarization and accompanying decrease in spike heights may also provide one mechanism for the classical finding that dopamine inhibits subcortically projecting mPFC neurons via D2Rs in vivo (Godbout et al., 1991; Pirot et al., 1992).
The dose-dependence of the quinpirole-induced afterdepolarization
Many studies have activated D2Rs using 10 μM quinpirole (Wang and Goldman-Rakic, 2004; Kreitzer and Malenka, 2005; Ramanathan et al., 2008; Sidiropoulou et al., 2009), similar to the doses used here (5 or 20 μM). Notably, Sidiropoulou et al. applied 10 μM quinpirole to layer V pyramidal neurons in PFC without activating D1Rs. Thus, we are confident that the quinpirole-induced afterdepolarization requires D2Rs, since it occurs selectively in D2R-expressing neurons, can be elicited by 5 μM quinpirole, and is eliminated by 5 μM (−)sulpiride, which selectively antagonizes D2Rs (Seeman and Van Tol, 1994).
So why is this afterdepolarization most prominent using 20 μM quinpirole, whereas quinpirole exerts distinct effects at lower doses (1–2 μM) (Tseng and O’Donnell, 2004; Tseng et al., 2008)? D2Rs couple to numerous signaling pathways, mediated by Gβγ, β-arrestins, and scaffolding proteins (Bonci and Hopf, 2005). D2R ligands possess functional selectivity for these pathways (Mailman, 2007), and the dose-dependence of quinpirole likely varies across these pathways (Urban et al., 2007). Notably, effects mediated by β-arrestin have a timecourse ~10 min (Ahn et al., 2004), similar to what we observed. Thus, although quinpirole may bind D2Rs at concentrations <1 μM, concentrations ~1–2 μM might recruit certain signaling pathways, while concentrations ~5–20 μM elicit distinct effects via non-canonical signaling pathways that are mediated by scaffolding proteins or receptor internalization. It will be important for future studies to elucidate these pathways.
Why haven’t previous studies observed the quinpirole-induced afterdepolarization?
The quinpirole-induced afterdepolarization is masked in quiescent slices, and could be unmasked via physiologic levels of synaptic stimulation or NMDA application (4–6 μM). One possible explanation is that the afterdepolarization originates in the dendrites, where D2Rs can be located (Negyessy and Goldman-Rakic, 2005). Thus, somatic recordings might not reveal D2R effects until dendritic excitability has been sufficiently enhanced by synaptic stimulation or NMDA. Indeed, while dopamine receptor effects have been extensively studied (Seamans and Yang, 2004), stimulating synapses using optogenetics might produce a more physiological state, thereby revealing phenomena as illustrated here.
Other studies have described distinct effects of quinpirole on layer V pyramidal neurons. Lower doses of quinpirole inhibit increases in pyramidal neuron excitability caused by AMPA or NMDA (Tseng and O’Donnell, 2004). The effects of quinpirole on responses to NMDA were mediated by D2Rs in inhibitory interneurons, rather than direct effects of D2Rs on pyramidal neurons. It is unclear whether this effect occurs under the same conditions or in the same neurons as the quinpirole-induced afterdepolarization, but this could be an important mechanism for regulating activity driven by the quinpirole-induced afterdepolarization. Indeed, inhibition might normally suppress this afterdepolarization, but pathological afterdepolarizations may emerge when prefrontal inhibition is compromised in schizophrenia or other conditions (Lewis et al., 2005).
Another study (Wang and Goldman-Rakic, 2004) found that doses of quinpirole similar to those used here promote bursting by thick-tufted layer V neurons in response to synaptic input when bicuculline and AP5 are present. We found that co-applying quinpirole, bicuculline, and AP5 also elicits an afterdepolarization that is blocked by nimodipine. Thus, the mechanism of the quinpirole-induced afterdepolarization may also underlie D2R-induced increases in bursting observed in that study.
The quinpirole-induced afterdepolarization depends on L-type Ca2+ channels and NMDA-Rs
We have shown that (1) D2R activation enhances plateau potentials mediated by Ca2+ and Ca2+-dependent currents, (2) the quinpirole-induced afterdepolarization involves both L-type channels and NMDA-Rs, and (3) chelating intracellular Ca2+ blocks the afterdepolarization. Thus, although D2Rs must enhance Ca2+ currents and/or Ca2+ dependent currents underlying the quinpirole-induced afterdepolarization, we cannot pinpoint the exact location of D2R action. Specifically, D2Rs might directly or indirectly enhance L-type currents, the accumulation of intracellular Ca2+, and/or ICAN. We found that in the presence of bicuculline, quinpirole-induced afterdepolarizations occur even after blocking NMDA-Rs. This suggests that NMDA-Rs facilitate, but are not absolutely necessary for these afterdepolarizations. Thus, D2Rs do not elicit the afterdepolarization via direct actions on NMDA-Rs. Of note, D1Rs in layer V pyramidal neurons in PFC can suppress L-type Ca2+ channel mediated potentials (Young and Yang, 2004). Since D1Rs and D2Rs often have opposing effects on overlapping signaling pathways, this suggests that D2Rs may enhance L-type Ca2+ channel mediated phenomena, contributing to the afterdepolarization we found.
L-type Ca2+ channels and NMDA-Rs produce other afterdepolarizations similar to those we have observed (Hounsgaard and Kiehn, 1989; Hernandez-Lopez et al., 1997; Hall and Delaney, 2002; Vergara et al., 2003; Lee and Tepper, 2007). Specifically, muscarinic receptors elicit similar afterdepolarizations in layer V of PFC (Haj-Dahmane and Andrade, 1998, 1999). Many of these afterdepolarizations also cause spike height rundown. Synergistic interactions between L-type Ca2+ channels and NMDA-Rs also produce regenerative depolarizations in pyramidal neuron dendrites (Schiller et al., 2000; Branco and Hausser, 2011). D2R activation could further amplify these interactions, by enhancing L-type Ca2+ currents and/or ICAN. In this way, D2Rs could profoundly enhance synaptic integration (Branco and Hausser, 2011).
L-type Ca2+ channels in PFC function and mental illness
L-type Ca2+ channels have been implicated in schizophrenia (Green et al., 2009; Bigos et al., 2010; Nyegaard et al., 2010; Ripke et al., 2011). A genetic polymorphism that increases L-type Ca2+ channel expression and schizophrenia risk also reduces prefrontal efficiency (Bigos et al., 2010), and L-type Ca2+ channel antagonists have shown promise for schizophrenia (Yamada et al., 1995; Yamada et al., 1996; Schwartz et al., 1997). L-type Ca2+ channels are also implicated in autism (Splawski et al., 2004) and bipolar disorder (Ferreira et al., 2008; Sklar et al., 2011). Despite these findings, specific mechanisms by which L-type Ca2+ channels modulate prefrontal function are lacking. Our results demonstrate an afterdepolarization through which L-type Ca2+ channels powerfully modulate prefrontal neurons. This may have consequences as described below.
The quinpirole-induced afterdepolarization in PFC function and mental illness
Layer V neurons exhibiting the quinpirole-induced afterdepolarization are well poised to affect the cognitive domains disrupted in schizophrenia. As we have shown, layer V pyramidal neurons that express D2Rs and exhibit the quinpirole-induced afterdepolarization correspond to a subpopulation (“type A”) that projects subcortically but not to contralateral PFC. In particular, our finding that D2Rs are restricted to type A neurons while D1Rs are expressed in type B neurons (which project cortically) as well, could explain the recent observation that blocking D1Rs in the frontal eyes fields modulates firing in visual cortex and brainstem, whereas activating D2Rs modulates activity in brainstem but not visual cortex (Noudoost and Moore, 2011). D2Rs might specifically enhance spiking in prefrontal neurons that trigger motor responses or corollary discharges (Robbins, 1990; Frith, 1995; Wang et al., 2004) – processes that are believed to depend on D2Rs and be abnormal in schizophrenia.
The quinpirole-induced afterdepolarization may also contribute to persistent reward related activity or the modulation of firing by the past history of reward (Histed et al., 2009; Bernacchia et al., 2011). Although the timescales of these phenomena may exceed those shown for the quinpirole-induced afterdepolarization, synaptic input interacts powerfully with the afterdepolarization. Thus, in vivo the quinpirole-induced afterdepolarization may amplify responses to weak synaptic input, producing additional firing and Ca2+ influx that sustain this afterdepolarization over longer timescales.
Finally, the quinpirole-induced afterdepolarization might increase the variability of PFC activity, facilitating adaptation to a changing environment (Durstewitz and Seamans, 2008; Durstewitz et al., 2010). Excessive D2R activation could produce noisy firing that is disconnected from external input and contributes to psychosis.
In summary, these findings define a novel mechanism, involving Ca2+ channels, through which D2Rs can powerfully regulate output from a defined subpopulation of prefrontal neurons. This mechanism is well-positioned to modulate prefrontal-dependent behaviors, including those disrupted in mental illness.
Acknowledgments
We acknowledge the advice and suggestions of Professors Robert C. Malenka, John L. Rubenstein, and John R. Huguenard on earlier versions of this work. K.D. is supported by the President and Provost of Stanford University, and by HHMI, CIRM, NSF, NIMH, NIDA, and the McKnight, Coulter, Kinetics, and Keck Foundations. V.S.S. is supported by the Staglin Family and International Mental Health Research Organization (IMHRO), R00 MH085946-02 from NIMH, the Simons Foundation for Autism Research, a Steve and Connie Lieber/NARSAD Young Investigator Award, and the Alfred P. Sloan Foundation.
Footnotes
CONFLICTS OF INTEREST: Stanford University has a pending patent application based on this work, which includes K.D. and V.S.S. as inventors.
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchia A, Seo H, Lee D, Wang XJ. A reservoir of time constants for memory traces in cortical neurons. Nat Neurosci. 2011;14:366–372. doi: 10.1038/nn.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G, Cherubini E, Marciani MG, Mercuri N, Stanzione P. Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Res. 1982;245:267–274. doi: 10.1016/0006-8993(82)90809-5. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR. Genetic Variation in CACNA1C Affects Brain Circuitries Related to Mental Illness. Archives of General Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Hopf FW. The dopamine D2 receptor: new surprises from an old friend. Neuron. 2005;47:335–338. doi: 10.1016/j.neuron.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Branco T, Hausser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron. 2011;69:885–892. doi: 10.1016/j.neuron.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav Brain Res. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MAR, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nature genetics. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Forscher P, Oxford GS. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985;85:743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. Functional imaging and cognitive abnormalities. Lancet. 1995;346:615–620. doi: 10.1016/s0140-6736(95)91441-2. [DOI] [PubMed] [Google Scholar]
- Godbout R, Mantz J, Pirot S, Glowinski J, Thierry AM. Inhibitory influence of the mesocortical dopaminergic neurons on their target cells: electrophysiological and pharmacological characterization. J Pharmacol Exp Ther. 1991;258:728–738. [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O’Donovan MC, Craddock N. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Molecular psychiatry. 2009;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. J Neurophysiol. 1998;80:1197–1210. doi: 10.1152/jn.1998.80.3.1197. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic receptors regulate two different calcium-dependent non-selective cation currents in rat prefrontal cortex. Eur J Neurosci. 1999;11:1973–1980. doi: 10.1046/j.1460-9568.1999.00612.x. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Delaney KR. Contribution of a calcium-activated non-specific conductance to NMDA receptor-mediated synaptic potentials in granule cells of the frog olfactory bulb. J Physiol. 2002;543:819–834. doi: 10.1113/jphysiol.2002.024638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63:244–253. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci. 2007;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton GO, Young AH, McQuade R, Fairchild G, Ingram CD, Gartside SE. Profound changes in dopaminergic neurotransmission in the prefrontal cortex in response to flattening of the diurnal glucocorticoid rhythm: implications for bipolar disorder. Neuropsychopharmacology. 2009;34:2265–2274. doi: 10.1038/npp.2009.53. [DOI] [PubMed] [Google Scholar]
- Minzer K, Lee O, Hong JJ, Singer HS. Increased prefrontal D2 protein in Tourette syndrome: a postmortem analysis of frontal cortex and striatum. J Neurol Sci. 2004;219:55–61. doi: 10.1016/j.jns.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488:464–475. doi: 10.1002/cne.20601. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM, Andersen PS, Nordentoft M, Werge T, Pedersen CB, Hougaard DM, Mortensen PB, Mors O, Borglum AD. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry. 2010;15:119–121. doi: 10.1038/mp.2009.69. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J Neurosci. 2008;28:11186–11195. doi: 10.1523/JNEUROSCI.1921-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirot S, Godbout R, Mantz J, Tassin JP, Glowinski J, Thierry AM. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience. 1992;49:857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Tkatch T, Atherton JF, Wilson CJ, Bevan MD. D2-like dopamine receptors modulate SKCa channel function in subthalamic nucleus neurons through inhibition of Cav2.2 channels. J Neurophysiol. 2008;99:442–459. doi: 10.1152/jn.00998.2007. [DOI] [PubMed] [Google Scholar]
- Ripke S, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16:391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Fay-McCarthy M, Kendrick K, Rosse RB, Deutsch SI. Effects of nifedipine, a calcium channel antagonist, on cognitive function in schizophrenic patients with tardive dyskinesia. Clin Neuropharmacol. 1997;20:364–370. doi: 10.1097/00002826-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Bunney BS. Pharmacological characterization of the receptor mediating electrophysiological responses to dopamine in the rat medial prefrontal cortex: a microiontophoretic study. J Pharmacol Exp Ther. 1989;248:1323–1333. [PubMed] [Google Scholar]
- Sheets PL, Suter BA, Kiritani T, Chan CS, Surmeier DJ, Shepherd GM. Corticospinal-specific HCN expression in mouse motor cortex: Ih-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J Neurophysiol. 2011 doi: 10.1152/jn.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou K, Lu FM, Fowler MA, Xiao R, Phillips C, Ozkan ED, Zhu MX, White FJ, Cooper DC. Dopamine modulates an mGluR5-mediated depolarization underlying prefrontal persistent activity. Nat Neurosci. 2009;12:190–199. doi: 10.1038/nn.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonic I, Gericke GS, Ott J, Weber JL. Identification of genetic markers associated with Gilles de la Tourette syndrome in an Afrikaner population. Am J Hum Genet. 1998;63:839–846. doi: 10.1086/302002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Huguenard JR. Inhibitory coupling specifically generates emergent gamma oscillations in diverse cell types. Proc Natl Acad Sci U S A. 2005;102:18638–18643. doi: 10.1073/pnas.0509291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TD, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, Lang AE, Strafella AP. Extrastriatal dopaminergic dysfunction in tourette syndrome. Ann Neurol. 2010;67:170–181. doi: 10.1002/ana.21809. [DOI] [PubMed] [Google Scholar]
- Stewart CV, Plenz D. Inverted-U profile of dopamine-NMDA-mediated spontaneous avalanche recurrence in superficial layers of rat prefrontal cortex. J Neurosci. 2006;26:8148–8159. doi: 10.1523/JNEUROSCI.0723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol. 2003;553:169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wang Y, Goldman-Rakic PS. D2 receptor regulation of synaptic burst firing in prefrontal cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2004;101:5093–5098. doi: 10.1073/pnas.0400954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ashikari I, Onishi K, Kanba S, Yagi G, Asai M. Effectiveness of nilvadipine in two cases of chronic schizophrenia. Psychiatry Clin Neurosci. 1995;49:237–238. doi: 10.1111/j.1440-1819.1995.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kanba S, Ashikari I, Ohnishi K, Yagi G, Asai M. Nilvadipine is effective for chronic schizophrenia in a double-blind placebo-controlled study. off. J Clin Psychopharmacol. 1996;16:437–439. doi: 10.1097/00004714-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon DY, Gause CD, Leckman JF, Singer HS. Frontal dopaminergic abnormality in Tourette syndrome: a postmortem analysis. J Neurol Sci. 2007;255:50–56. doi: 10.1016/j.jns.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Young CE, Yang CR. Dopamine D1/D5 receptor modulates state-dependent switching of soma-dendritic Ca2+ potentials via differential protein kinase A and C activation in rat prefrontal cortical neurons. J Neurosci. 2004;24:8–23. doi: 10.1523/JNEUROSCI.1650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadee W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]