Abstract
Although exclusively breastfed infants are at increased risk of vitamin D (vit D) deficiency if vit D supplementation is lacking and sun exposure is limited, assessment of both risk factors in the first year of life is lacking. We evaluated contribution of vit D intake and sunlight exposure to vit D status in 120 healthy, breastfeeding mother-infant dyads, who were followed for 1 year. Vitamin D intake and skin sunlight exposure were evaluated using questionnaires. Serum 25(OH)D, PTH and ALP levels were determined postnatally in mothers at 4 weeks and in infants at 4, 26 and 52 weeks. Vitamin D supplementation was low (<20%) and sunlight exposure was common (93%) in study infants. At 4 weeks, 17% of mothers were vit D deficient (<50 nmol/L) and 49% were insufficient (50-<75 nmol/L), while 18% of infants were severely vit D deficient (<25 nmol/L) and 77% were deficient (<50 nmol/L). At 26 weeks, winter/spring birth season and shorter duration of months of exclusive breastfeeding were protective of vit D deficiency in infants. Vitamin D deficiency in infants decreased to 12% at 52 weeks with sunlight exposure. Serum PTH levels were significantly higher in severely vit D deficient than sufficient infants. Vitamin D deficiency was widespread in early postpartum breastfeeding mothers and infants and declined to 1 in 8 infants at 52 weeks due mostly to sunshine exposure. When sunlight exposure is limited or restricted intensified vit D supplementation of breastfeeding mothers and infants is needed to improve vit D status.
Keywords: Vitamin D deficiency, breastfeeding, sunlight exposure, infants, mothers
Introduction
Severe vitamin D (vit D) deficiency presenting as rickets is a public health problem worldwide (Holick 2006, Thacher et al. 2006); and there are increasing reports of its occurrence especially among breastfed infants in minority groups in the United States and elsewhere (Thacher et al. 2006, Dawodu et al. 2005, Strand et al. 2007, Weisberg et al. 2004). A recent study found that one-third of infants with vit D deficiency who had radiographs performed had evidence of bone under-mineralization (Gordon et al. 2008). In addition, vit D insufficiency and low vit D intake in childhood has been linked to increased risk of acute lower respiratory tract infections (McNally et al. 2009, Wayse et al. 2004) and autoimmune diseases such as type 1 diabetes (Hypponen et al. 2001). Vitamin D insufficiency in adults is reported to be associated with increased risk of autoimmune diseases, certain types of cancer, cardiovascular and infectious diseases (Holick 2007).
Breastfeeding without infant vit D supplementation, a lack of sunlight exposure and maternal vit D deficiency are reported as important risk factors for vit D deficiency rickets (Dawodu et al. 2005, Holick 2006, Strand et al. 2007, Thacher et al. 2006, Weisberg et al. 2004). The American Academy of Pediatrics (AAP) recommends that children younger than 6 months of age avoid direct sunlight because of concern about skin cancers in later life, and thus, should receive vit D supplementation to prevent vit D deficiency (AAP 1999, Wagner & Greer 2008). However, compliance with the recommendation for vit D supplementation appears low. Previous excellent studies (Greer et al. 1982, Ho et al. 1985, Specker & Tsang 1987) performed over 2 decades ago showed relationships among sun exposure, vit D supplementation and maternal vit D on infant vit D status. However, despite increasing reports of vit D deficiency in breastfeeding infants, recent studies that attempted to quantify the impact of sun exposure, vit D supplementation, and maternal vit D status in relation to vit D status in breastfeeding infants during infancy (Andiran et al. 2002, Challa et al. 2005, Dawodu et al. 2003) are scarce. Such information is of public health importance because it could help identify and guide appropriate intervention strategies to avoid vit D deficiency in breastfeeding mothers and infants.
We conducted this study to investigate the prevalence and relationship of vit D deficiency in breastfeeding mother-infant pairs in Cincinnati, Ohio and the effect of maternal vit D status, infant vit D supplementation, and sun exposure on infant vit D status during the first year of life.
Methods
Subjects
One-hundred and twenty breast feeding mother-infant pairs enrolled in this longitudinal observational study were participants in an International Human Milk Research Collaborative to examine the association between mothers’ nutritional status, human milk factors and child health outcomes during the first 2 years of life. The protocol of the parent study was approved by Cincinnati Children’s Hospital Institutional Review Board and the mothers gave written informed consent. The sample size of the parent study was determined to assess the association between bioactive factors in human milk and growth and the prevalence of respiratory infections and diarrhea during the first 2 years of life. Subjects recruited were healthy, singleton term infants (≥37 weeks) whose mothers were aged 18–49 years and planned to provide at least 75% of feedings from breast milk for at least 3 months. Infants who were born prematurely (<37 weeks) or mothers with health problems that could interfere with breastfeeding were excluded. Healthy mothers were recruited from mothers who delivered at The Christ Hospital in Cincinnati, OH, between March 2007 and July 2008. This report concerns the prevalence and risk factors of vit D deficiency in mother-infant pairs at 4 weeks postpartum (baseline), and in the infants at longitudinal follow-up during the first year of life.
Study Design
At 2 weeks post-partum, a registered nurse visited the family home for enrollment. Post-partum visits at 4, 13, 26 and 52 weeks of life took place at the Cincinnati Center for Clinical Research to complete baseline demographic data and follow-up questionnaires. Blood samples were collected by venipuncture from the mother at 4 weeks post-partum and at weeks 4, 26 and 52 from the infants. A 24-hour dietary recall questionnaire, including dietary supplements was administered three times between weeks 4 and 13 for the mother. Nutrition Data Systems for Research (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) was used to assess the micronutrient intake from foods reported in the dietary recall interview, while maternal vit D supplementation was assessed from the list of vitamin supplements intake reported during the interview. The total maternal vit D intake included both dietary and supplemented sources of vit D. Mothers also indicated the intake of any vitamins or medications provided to the infant within the past 24 hours during weekly phone interviews beginning at 2 weeks postpartum. Maternal report of giving the infant a multivitamin, vit D supplement or cod liver oil was included as evidence of infant vit D supplementation.
Sun exposure behavior questionnaires (Table 1) were administered by a registered nurse to mother and her infant at weeks 4, 26 and 52. The questionnaire is a modification of previous questionnaires used in assessing skin exposure to sunlight in adults and infants (Barger-Lux & Heaney 2002, Dawodu et al. 1998, Specker et al. 1985b). Table 1 also provides the method for calculating the total percent of body surface area (% BSA) exposed while outdoors for mothers and infants. A sun exposure index was estimated by multiplying the % BSA exposure by hours of sunlight exposure per week. Sun exposure index has been shown to correlate with vit D status in adults and children (Barger-Lux & Heaney 2002, Dawodu et al. 1998, Specker et al. 1985b). Collection of data on sunscreen use began after the study was underway and was thus incomplete.
Table 1.
Calculation of percent body surface area (% BSA) exposed to sunlighta
| Usual Outdoor Daylight Attire in Past Week: | % BSA assigned for: | |||
|---|---|---|---|---|
|
| ||||
| Body part | Type of covering | Response | Mother | Infant |
| Head | Hat, cap, or head scarf | Yes | 0 | 0 |
| No | 3 | 12 | ||
|
| ||||
| Neck | Clothing or scarf | Yes | 0 | 0 |
| No | 2 | 2 | ||
|
| ||||
| Face | Scarf | Yes | 0 | 0 |
| No | 4 | 7 | ||
|
| ||||
| Arms | Length of sleeves | Long | 0 | 0 |
| Short | 6 | 6 | ||
| Sleeveless | 14 | 14 | ||
|
| ||||
| Hands | Gloves | Yes | 0 | 0 |
| No | 5 | 5 | ||
|
| ||||
| Legs | Length of pants or dress | Long | 0 | 0 |
| Short (below knee) | 7 | 5 | ||
| Short (knee length) | 14 | 10 | ||
| Short (mid-thigh) | 23 | 16 | ||
|
| ||||
| Feet | Amount of coverage | Covered | 0 | 0 |
| Barefoot or sandals | 7 | 7 | ||
Total % BSA exposed to sunlight calculated as the sum of % BSA associated with subject’s responses for each body part.
Laboratory Tests
Serum concentrations of 25-hydroxyvitamin D [25(OH)D] were determined by radioimmunoassay (DiaSorin, Stillwater, Minnesota) to assess the vit D status of mothers and infants. The intra-assay and inter-assay coefficient of variation for 25(OH)D measurement were 4% and 11% respectively. In the mothers, vit D deficiency was defined as serum 25(OH)D <50 nmol/L (Holick 2007, Institute of Medicine 2011, Holick et al. 2011) while 25(OH)D between 50–75 nmol/L was considered vit D insufficient (Bischoff-Ferrari et al. 2006, Holick 2007). Vitamin D deficiency was also defined as serum 25(OH)D <50 nmol/L in the infants (Wagner & Greer 2008, Holick et al. 2011). For this study, serum 25(OH)D levels <25 nmol/L, consistent with higher risk of rickets (Dawodu et al. 2005, Institute of Medicine 2011) was considered as severe vit D deficiency in the infants. Serum intact parathyroid hormone (PTH) was measured by immunoradiometric assay (DiaSorin). The normal adult range for serum PTH in our laboratory is 13–54 pg/mL. The range for infants using this assay is not established and the adult range was adopted as in other studies (Liang et al. 2010, Zeghoud et al. 1997). In addition, bone serum alkaline phosphatase (ALP) was measured by enzyme immunoassay (Quidel Microvue BAP, Santa Clara, CA, USA), because significantly elevated levels have been reported in breastfed infants with severe vit D deficiency (Ziegler et al. 2006). The normal range using the assay is 6–34 IU/L for adult and 8–142 IU/L for children (0–2 yrs).
Statistical analysis
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). For the baseline analysis, we estimated the prevalence of the different categories of vit D status and assessed the contribution of the selected key risk factors to vit D deficiency in mothers and in infants using univariate and multivariate ANOVA. Comparisons of mean 25(OH)D concentrations for different categories of vit D status were made using ANOVA with Tukey-Kramer adjustments for multiple comparisons. Differences in percentages of categories of vit D status were compared using χ2 or Fisher’s exact test. Pearson correlations were used for correlation analyses.
Seasons of birth were categorized as Winter/Spring (October-March) and Summer/Fall (April-September). At each time point, we conducted univariate ANOVA or linear regression analysis and selected characteristics with a univariate p<0.10 to consider for repeated measures multivariate analysis of vit D status. ANCOVA with backward selection was employed to determine which subset of characteristics was most predictive of vit D status. The longitudinal analysis of factors affecting infant vit D status was assessed using repeated measures (ANCOVA), adjusting for key covariates and multiple measurements per infant over time. Infants’ longitudinal changes in vit D status across time were tested using the paired McNemar test of symmetry.
Results
Ninety-nine mothers were non-Hispanic white, 17 African American and 4 identified as other ethnic groups. The mean (SD) age was 31.5 (5.2). Eighty-two mothers had completed at least 4 years of college education. A significantly higher proportion (75%) of non-Hispanic white mothers had completed at least 4 years of college education compared with 35% of African American mothers (p≤0.001).
Vitamin D Status of Mothers and Infants
Of the 120 mother-infant pairs enrolled in the study, serum 25(OH)D levels were available in 119 mothers and in 87 (73%) of the infants at 4 weeks postpartum. There were no significant differences in the demographics, sun exposure behaviors, and maternal vit D status between mother-infant pairs with and without 4 week serum 25(OH)D levels (results not shown). We examined predictors of vit D status in the mothers (Table 2) and infants (Table 3) using categories of serum 25(OH)D concentrations. At 4 weeks postpartum, a total of 79 (66%) mothers were either vit D insufficient (50–75 nmol/L, n=59) or deficient (<50 nmol/L, n=20). In the mothers, black race and winter/spring season were significant factors associated with higher percentage of vit D deficiency in univariate analysis (Table 2). African American mothers were 9 times (OR [95% CI]: 9.3 [3.0, 29.1]) much more likely to be vit D deficient than mothers of other races.
Table 2.
Predictors of Maternal Vitamin D Status at 4 Weeks Postpartum
| Maternal Serum 25(OH)D Concentration Status | |||||||
|---|---|---|---|---|---|---|---|
| Number (%) | |||||||
| Variable | N | Serum 25(OH)D (Mean ± SD) | p-value for ANOVA | < 50 nmol/L | 50 – <75nmol/L | ≥ 75 nmol/L | p-value for difference* |
| All subjects vitamin D status | 119 | 70.2 ± 23.2 | 40.4 ± 9.4 | 63.6 ± 7.0 | 94.9 ± 19.2 | ||
| Ethnicity | |||||||
| White | 98 | 73.2 ± 21.5 | < 0.001 | 11 (11%) | 49 (50%) | 38 (39%) | < 0.001 |
| African-American | 17 | 49.1 ± 18.1 | 9 (53%) | 7 (41%) | 1 (6%) | ||
| Other | 4 | 85.9 ± 37.9 | 0 (0%) | 3 (75%) | 1 (25%) | ||
| Maternal Education Status | |||||||
| Completed 4 Year College | 82 | 71.8 ± 22.7 | 0.28 | 11 (13%) | 42 (51%) | 29 (35%) | 0.34 |
| Did Not Complete 4 Year College | 37 | 66.8 ± 24.3 | 9 (24%) | 17 (46%) | 11 (30%) | ||
| Maternal BMI Class | |||||||
| Not Obese (BMI < 30) | 77 | 72.7 ± 22.9 | 0.12 | 9 (12%) | 39 (51%) | 29 (38%) | 0.10 |
| Obese (BMI ≥ 30) | 42 | 65.8 ± 23.5 | 11 (26%) | 20 (48%) | 11 (26%) | ||
| Maternal Vit D Supplementation | |||||||
| Any Supplementation | 93 | 72.7 ± 21.1 | 0.08 | 11 (12%) | 47 (51%) | 35 (38%) | 0.20 |
| No Supplementation | 14 | 61.2 ± 28.6 | 4 (29%) | 7 (50%) | 3 (21%) | ||
| Season | |||||||
| Winter/Spring | 41 | 60.9 ± 15.0 | 0.001 | 11 (27%) | 23 (56%) | 7 (17%) | 0.009 |
| Summer/Fall | 78 | 75.1 ± 25.3 | 9 (12%) | 36 (46%) | 33 (42%) | ||
| Sun Exposure Time | |||||||
| Lowest quartile (< 1.83 hrs) | 29 | 69.8 ± 17.4 | 0.44 | 2 (7%) | 18 (62%) | 9 (31%) | 0.38 |
| Highest quartile (> 8.54 hrs) | 30 | 65.9 ± 21.2 | 6 (20%) | 16 (53%) | 8 (27%) | ||
| Sun Exposure (BSA) | |||||||
| Lowest quartile (< 19%) | 29 | 65.7 ± 20.6 | 0.13 | 6 (21%) | 15 (52%) | 8 (28%) | 0.35 |
| Highest quartile (> 49%) | 26 | 75.3 ± 25.1 | 3 (12%) | 11 (42%) | 12 (46%) | ||
| Sun Index | |||||||
| Lowest quartile (< 42.5) | 29 | 68.1 ± 17.8 | 0.47 | 3 (10%) | 18 (62%) | 8 (28%) | 0.67 |
| Highest quartile (> 338.4) | 30 | 64.7 ± 18.7 | 6 (20%) | 16 (53%) | 8 (27%) | ||
| Sunscreen Usage | |||||||
| Any sunscreen usage | 18 | 72.9 ± 25.4 | 0.60 | 3 (17%) | 7 (39%) | 8 (44%) | 0.73 |
| No sunscreen usage | 28 | 69.3 ± 20.7 | 5 (18%) | 14 (50%) | 9 (32%) | ||
For vit D level, mean comparisons made with ANOVA. For tests of proportions, differences were tested using chi-square or Fisher’s Exact test, as appropriate.
Table 3.
Predictors of Infant Vitamin D Status at 4 Weeks of Age
| Infants’ Serum 25(OH)D Concentration Status | |||||||
|---|---|---|---|---|---|---|---|
| Number (%) | |||||||
| Variables | N | Serum 25(OH)D (Mean ± SD) | p-value for ANOVA | < 25 nmol/L | 25 – <50 nmol/L | ≥ 50 nmol/L | p-value for difference* |
| All subjects vitamin D status | 87 | 41.0 ± 16.3 | 18.7 ± 3.5 | 39.2 ± 7.0 | 63.5 ± 10.1 | ||
| Ethnicity | |||||||
| White | 69 | 43.5 ± 15.6 | 0.008 | 8 (12%) | 43 (62%) | 18 (26%) | 0.005 |
| African-American | 9 | 26.9 ± 14.4 | 6 (67%) | 2 (22%) | 1 (11%) | ||
| Other | 9 | 35.7 ± 16.1 | 2 (22%) | 6 (67%) | 1 (11%) | ||
| Maternal Education Status | |||||||
| Completed 4 Year College | 58 | 42.4 ± 17.1 | 0.25 | 9 (16%) | 33 (57%) | 16 (28%) | 0.29 |
| Did Not Complete 4 Year College | 29 | 38.1 ± 14.5 | 7 (24%) | 18 (62%) | 4 (14%) | ||
| Maternal Vit D Supplementation | |||||||
| Any Supplementation | 71 | 40.0 ± 15.7 | 0.12 | 13 (18%) | 43 (61%) | 15 (21%) | 0.54 |
| No Supplementation | 11 | 48.2 ± 17.4 | 1 (9%) | 6 (55%) | 4 (36%) | ||
| Infant Vit D Supplementation | |||||||
| Supplemented Prior to 4 Weeks of Age | 3 | 51.5 ± 34.0 | 0.26 | 1 (33%) | 0 (0%) | 2 (67%) | 0.07 |
| Not Supplemented Prior to 4 Weeks of Age | 84 | 40.6 ± 15.6 | 15 (18%) | 51 (61%) | 18 (21%) | ||
| Season | |||||||
| Winter/Spring | 31 | 34.0 ± 14.7 | 0.002 | 10 (32%) | 17 (55%) | 4 (13%) | 0.03 |
| Summer/Fall | 56 | 44.9 ± 15.9 | 6 (11%) | 34 (61%) | 16 (29%) | ||
| Sun Exposure Time | |||||||
| Lowest quartile (< 0.67 hrs) | 22 | 34.1 ± 12.6 | 0.003 | 6 (27%) | 14 (64%) | 2 (9%) | 0.03 |
| Highest quartile (> 5.83 hrs) | 19 | 48.3 ± 16.3 | 1 (5%) | 10 (53%) | 8 (42%) | ||
| Sun Exposure (BSA) | |||||||
| Lowest quartile (< 12%) | 22 | 36.4 ± 16.1 | 0.04 | 7 (32%) | 11 (50%) | 4 (18%) | 0.03 |
| Highest quartile (> 43%) | 17 | 46.7 ± 12.8 | 0 (0%) | 13 (77%) | 4 (24%) | ||
| Sun Index | |||||||
| Lowest quartile (< 10.75) | 22 | 32.4 ± 12.8 | < 0.001 | 8 (36%) | 12 (55%) | 2 (9%) | 0.01 |
| Highest quartile (> 161.5) | 21 | 49.0 ± 14.7 | 1 (5%) | 12 (57%) | 8 (38%) | ||
| Sunscreen Usage | |||||||
| Any sunscreen usage | 1 | 60.0 ± N/A | 0.13 | 0 (0%) | 0 (0%) | 1 (100%) | 0.21 |
| No sunscreen usage | 32 | 35.6 ± 15.6 | 9 (28%) | 17 (53%) | 6 (19%) | ||
| Maternal Vit D Status | |||||||
| Deficient (< 50 nmol/L) | 15 | 35.5 ± 18.7 | 0.08 | 6 (40%) | 5 (33%) | 4 (27%) | 0.04 |
| Insufficient (50 – <75 nmol/L) | 45 | 39.6 ± 15.8 | 8 (18%) | 30 (67%) | 7 (16%) | ||
| Sufficient (≥ 75 nmol/L) | 26 | 46.7 ± 15.0 | 2 (8%) | 15 (58%) | 9 (35%) | ||
For vit D level, mean comparisons made with ANOVA. For tests of proportions, differences were tested using chi-square or Fisher’s Exact test, as appropriate.
Of the 87 infants, a total of 67 (77%) were vit D deficient (serum 25(OH)D <50 nmol/L), of which fifty-one had 25 (OH)D levels of 25–50 nmol/L and 16 were severely deficient (serum 25(OH)D <25 nmol/L) at 4 weeks of age. In univariate analysis, black race (p=0.005), maternal vit D status (p=0.04) winter/spring birth season (p=0.03), lowest quartile sun exposure time (p=0.03), %BSA exposure (p=0.03) and sun index (p<0.001) were associated with higher prevalence of vit D deficiency and severe vit D deficiency (Table 3). African American infants were 13 times (OR [95% CI]: 13.6 [2.9, 63.2]) much more likely to be severely vit D deficient than other infants.
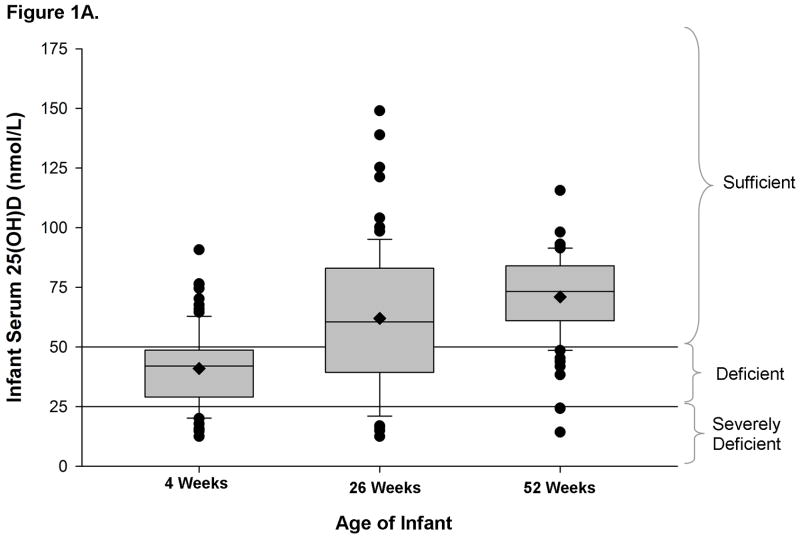
Unadjusted mean serum 25(OH)D levels increased from 41.0 nmol/L at 4 weeks (N=87) to 62.1 nmol/L at 26 weeks (N=77) and to 71.0 nmol/L at 52 weeks (N=80); (all pairwise p<0.02 after adjustment for multiple comparisons; Figure 1A). In repeated measures longitudinal analysis, mean serum 25(OH)D levels (±standard error) similarly increased from 45.0 (±3.8) nmol/L at 4 weeks to 72.6 (±4.0) nmol/L at 26 weeks to 80.3 (±4.2) nmol/L at 52 weeks (all pairwise p<0.02), adjusting for the infant characteristics of race, exclusive breastfeeding, formula use, infant vit D supplementation, season of measurement and an interaction between season and infant age, and the maternal characteristics of education level, vit D sufficiency and supplementation, and % BSA exposed to sunlight.
Figure 1.
Figure 1A. Infant serum 25(OH)D (nmol/L) measurements at 4, 26, and 52 weeks of age. Box indicates median and interquartile range; whiskers delineate 90th and 10th percentiles, with individual values outside those percentiles denoted by closed circles. Diamonds within the boxes designate the unadjusted mean levels. Horizontal lines show thresholds for vitamin D deficiency and severe deficiency.
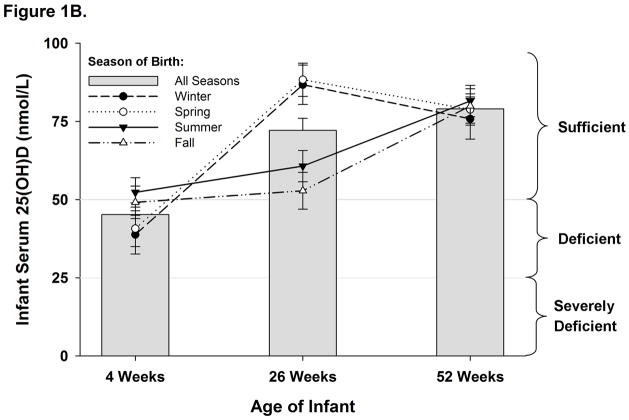
Figure 1B. Adjusted mean ± standard error of infants’ serum 25(OH)D (nmol/L) by age for each birth season. Means are adjusted for race, maternal education level, maternal Vitamin D sufficiency, maternal Vit D supplementation, maternal %BSA exposed to sunlight, exclusive breastfeeding, infant Vitamin D supplementation, and formula use. Lines represent the trajectory of serum 25(OH)D (nmol/L) over time for infants born during each season. Horizontal lines show thresholds for vitamin D deficiency and severe deficiency.
The changes in infants’ adjusted serum 25(OH)D levels reflected both the season of birth and age (Figure 1B). Infants’ 25(OH)D levels did not differ by season of birth at either 4 weeks or 52 weeks of age (all pairwise p>0.7). However, infant 25(OH)D levels were higher for those who reached 26 weeks during summer or fall (i.e. born in winter/spring) than those who reached 26 weeks during winter or spring (i.e. born in summer/fall; all pairwise p<0.02).
Conversely, the prevalence of vit D deficiency declined from 77% at 4 weeks to 31% at 26 weeks and 12.5% at 52 weeks, while the prevalence of severe vit D deficiency declined from 18% at 4 weeks to 13% at 26 weeks and 4% at 52 weeks. The decline in deficiency was stronger between 4 weeks and both 26 and 52 (McNemar test p≤0.001) than between 26 and 52 weeks (McNemar test p=0.054).
Infant milk source changed during the follow-up and impacted vit D status. Fifteen out of 120 (12.5%) infants were fed formula at 4 weeks, while 31 out of 96 (32%) used formula at 26 weeks. At 26 weeks, any formula usage was associated with higher serum 25(OH)D levels (adjusted mean ± SE: 83±5.3 nmol/L vs. 63±4.3 nmol/L, p<0.001).
Vitamin D Intake
Ninety-three of the 107 (87%) mothers with data on dietary intake reported taking vit D supplements. The median interquartile range vit D intake from diet and supplement combined was 540 IU/day (420, 640). There were no ethnic differences in daily vit D intake. Maternal vit D intake was not associated with vit D status in this cohort (p=0.59). Mothers’ report of vit D supplementation compared with no supplementation showed only a trend to higher serum 25(OH)D (p=0.08) and did not impact her category of vit D status at 4 weeks postpartum (Table 2).
Only 32 (27%) of the infants ever received vit D supplementation. Supplementation was initiated before 4 weeks of age in only 6 (5%) and in 19% by 6 months of age. In view of the small number of infants supplemented with vit D at 4 weeks of age, we could not make a valid assessment of the effect of supplementation on infant vit D status.
Maternal vit D supplementation status in the first month postpartum did not affect the infant vit D status (Table 3).
Sun Exposure Behaviors
Sun exposure behaviors in mothers and infants varied by season and age of infant (Table 4). At 4 weeks post-partum, mothers reported significantly more hours of direct sunlight exposure week-1 in spring than winter. Median % BSA exposed outdoors and sun index were lower in winter than in the other seasons (Table 4). Maternal sun exposure time, % BSA exposed and sun index did not affect her category of vit D status (Table 2). In this study, there was no interaction between race and maternal sun index and serum 25(OH)D concentrations.
Table 4.
Sunlight Exposure for Mothers and Infants by Season of Measurement
| N | Winter | Spring | Summer | Fall | P-Value | |||
|---|---|---|---|---|---|---|---|---|
| Mother | 4 weeks | % BSA | 120 | 10.0 (7.0, 14.0)A | 34.0 (27.0, 50.0)BC | 43.0 (34.0, 50.0)B | 27.0 (14.0, 39.0)C | <0.001 |
| Hours | 120 | 1.7 (0.7, 6.0)A | 7.5 (3.4, 12.5)B | 4.3 (2.1, 8.0)AB | 4.0 (2.1, 8.0)AB | 0.02 | ||
| Sun Index | 120 | 18.8 (6.0, 54.0)A | 340 (84.0, 544)B | 198 (90.6, 384)B | 108 (29.2, 188)B | <0.001 | ||
| Infant | 4 weeks | % BSA | 118 | 3.5 (0.0, 9.0)A | 21.0 (12.0, 33.0)B | 42.0 (32.0, 48.0)C | 26.0 (9.0, 42.0)B | <0.001 |
| Hours | 120 | 1.3 (0.3, 3.8) | 3.8 (1.8, 7.0) | 2.3 (0.7, 4.3) | 3.8 (0.8, 6.0) | 0.07 | ||
| Sun Index | 118 | 1.2 (0.0, 12.0)A | 54.0 (27.0, 236)B | 63.8 (27.2, 220)B | 50.0 (15.2, 175)B | <0.001 | ||
| 26 weeks | % BSA | 91 | 9.0 (5.0, 14.0)A | 14.0 (12.0, 24.0)AC | 37.0 (30.0, 55.0)B | 26.0 (14.0, 32.0)C | <0.001 | |
| Hours | 93 | 1.0 (0.3, 2.2)A | 2.0 (1.0, 4.5)A | 14.0 (7.5, 16.5)B | 2.5 (1.0, 5.8)A | <0.001 | ||
| Sun Index | 91 | 5.2 (0.0, 19.5)A | 40.0 (8.3, 94.5)AC | 473 (278, 880)B | 44.0 (24.0, 165)C | <0.001 | ||
| 52 weeks | % BSA | 87 | 9.0 (7.0, 12.0)A | 26.0 (14.0, 32.0)B | 49.0 (43.0, 55.0)C | 35.5 (26.0, 44.0)B | <0.001 | |
| Hours | 92 | 1.8 (1.2, 7.0)A | 5.0 (1.7, 11.2)A | 10.5 (5.4, 14.0)B | 6.0 (3.4, 8.9)AB | 0.003 | ||
| Sun Index | 87 | 24.0 (0.0, 74.0)A | 104 (33.8, 271)B | 528.5 (224, 753)C | 272 (76.3, 457)B | <0.001 |
Median (Interquartile Range) is reported. Median differences were tested using the Kruskal-Wallis test, and pairwise p-values were calculated using the Tukey-Kramer adjustment for multiple comparisons. Medians with the same superscript in a given row are significantly different. Sun Index is defined as the product of %BSA and weekly hours of sun exposure.
A total of 93%, 92% and 96% of the infants were reportedly exposed to direct sunlight at 4, 26 and 52 weeks respectively. The median duration of reported direct sunlight exposure/week by 4-week old infants did not vary significantly by season, but the median % BSA was higher in summer than other seasons (p< 0.001). The sun index was lower in winter than other seasons (Table 4). There were positive correlations between mothers and infants in the duration of sun exposure (r=0.47, p<0.001) and % BSA exposed while outdoors (r=0.60, p< 0.001). There were no ethnic differences in the sun exposure behaviors. As noted before, duration of sun exposure, % BSA exposed and sun index were significant determinants of infants’ vitamin D status in univariate analysis (Table 3).
Of 47 mothers with information at 4 weeks postpartum on sunscreen use, 18 (38%) reported using sunscreen and of those 95% used a sun protection factor (SPF) >8. In contrast, of these 47 mothers only one reported using sunscreen on her infant at 4 weeks of age while 31 (34%) of 92 mothers reported using sunscreen on their infants at 52 weeks of age. In this subset, sunscreen usage did not significantly affect maternal or infant vit D levels (Tables 2 and 3).
Independent Predictors of Vitamin D Sufficiency
Based on the significant factors on univariate analysis, multivariable models were constructed at each time point to determine independent predictors of maternal and infant vit D status. At 4 weeks postpartum, vit D sufficient mothers (serum 25(OH)D ≥75 nmol/L) were significantly more likely to be white (OR [95% CI]: 8.3 [1.8, 38.8], p=0.007) and to be measured during the summer/fall seasons (4.5 [1.7,11.8], p=0.002) compared to vit D insufficient or deficient mothers.
In infants, higher 25(OH)D levels at 4 weeks of age were independently associated with non-Hispanic white race (β±SE: 14.3 ± 3.9 nmol/L, p=0.001) and summer/fall season of birth (β±SE: 12.6 ± 3.3 nmol/L, p=0.001). At 26 weeks of age, independent predictors of infant vit D sufficiency were being born in winter/spring seasons (OR [95% CI]: 10.7 [2.6,43.9], p<0.001) and shorter duration of exclusive breastfeeding (OR [95% CI]: 0.6 [0.4,0.9] per month of exclusive breastfeeding, p=0.003). At 52 weeks of age, only 4-week 25(OH)D levels were significantly associated with vit D sufficiency. However, excluding 4-week 25 (OH)D levels as a potential covariate, current weekly hours of sun exposure (OR [95% CI]: 1.25 [1.03, 1.51] per hour of sun exposure, p=0.01) was the only other independent predictor of vit D sufficiency in infants at 52 weeks.
Serum PTH and ALP Concentrations and Vitamin D Status
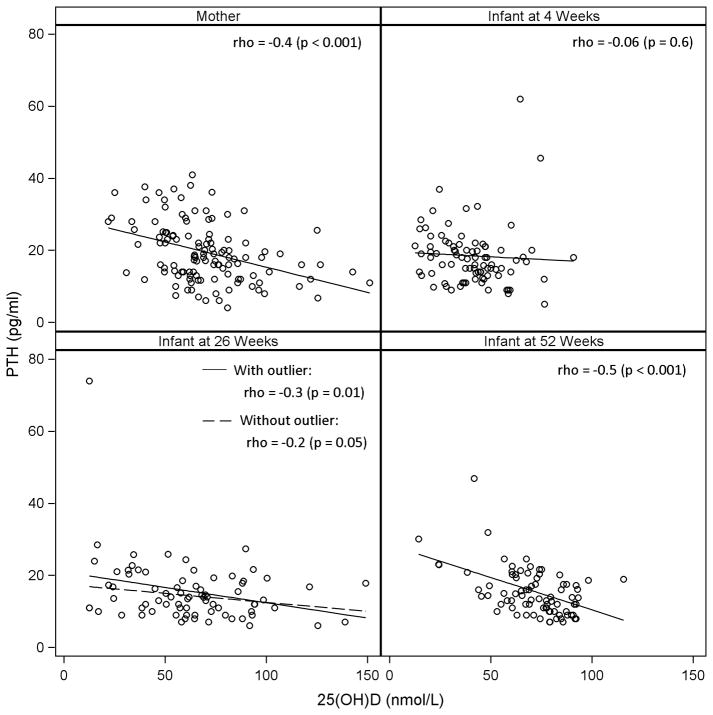
There were negative correlations between serum 25(OH)D levels and PTH values in mothers (r= −0.4, p<0.001) at 4 weeks (Figure 2) postpartum with the highest PTH levels in vit D deficient mothers (25.6 ± 8.2), followed by insufficient mothers (20.1 ± 8.2), and lowest in vit D sufficient mothers (15.2 ± 6.0) (all p < 0.02). There was no significant correlation between serum ALP and maternal 25(OH)D (r= −0.17, p=0.06) and the ALP levels in mothers with different categories of vit D status were not different (p = 0.3).
Figure 2.
Serum 25(OH)D levels and intact parathyroid hormone levels for mothers at 4 weeks post-partum and for infants at 4, 26, and 52 weeks of age. Pearson correlation coefficients and p-values are reported. Solid lines are regression lines for the data. Dashed line for infants at 26 weeks excludes one outlying value from the regression calculation.
Serum 25(OH)D levels also correlated with PTH values in infants at 26 (r= −0.3, p=0.01) and 52 weeks (r= −0.5, p<0.001), but not at 4 weeks (p=0.56) (Figure 2). Serum 25(OH)D concentrations showed negative weak correlations with serum ALP values (r= −0.16, p=0.02). Neither PTH nor ALP levels demonstrated a non-linear relationship with 25(OH)D levels (data not shown). The relationships between categories of infant vit D status and serum PTH and ALP levels during the first year follow-up are summarized in Table 5. Serum PTH levels were significantly higher only in severely vit D deficient compared to sufficient infants at 26 weeks. The mean ALP levels did not differ significantly by categories of vit D status in infants.
Table 5.
Longitudinal Assessment of Infant Serum PTH and ALP by Vitamin D Status
| Measurement | Age | n | Vitamin D Status | Omnibus p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Severely Deficient (< 25 nmol/L) | Deficient (25 –<50 nmol/L) | Sufficient (≥ 50 nmol/L) | |||||||
| n | Value | n | Value | n | Value | ||||
| PTH (pg/mL) | 4 Weeks | 87 | 16 | 21.3 ± 1.9 | 51 | 17.1 ± 1.1 | 20 | 18.5 ± 1.7 | 0.04 |
| 26 Weeks | 76 | 9 | 22.9 ± 2.5A | 14 | 16.7 ± 2.0AB | 53 | 13.6 ± 1.0B | ||
| 52 Weeks | 80 | 3 | 25.3 ± 4.3 | 7 | 23.0 ± 2.8 | 70 | 14.1 ± 0.9 | ||
| ALP (IU/L) | 4 Weeks | 66 | 12 | 223 ± 44 | 38 | 279 ± 24 | 16 | 199 ± 38 | 0.78 |
| 26 Weeks | 72 | 9 | 115 ± 50 | 12 | 180 ± 42 | 51 | 140 ± 21 | ||
| 52 Weeks | 76 | 3 | 166 ± 87 | 7 | 167 ± 57 | 66 | 184 ± 18 | ||
Least square means ± standard errors are reported. Mean differences were tested using repeated measures ANOVA, and pairwise p-values were calculated using the Tukey-Kramer adjustment for multiple comparisons. Means in a row with different superscript letters are significantly different.
Discussions
In this prospective cohort study, vit D deficiency and insufficiency is widespread in breastfeeding mothers and their infants at 4 weeks in Cincinnati. Based on available evidence, the AAP, Institute of Medicine and the Endocrine Society guidelines (Institute of Medicine 2011, Wagner & Greer 2008, Holick et al. 2011) recommend serum 25(OH)D concentrations ≥50 nmol/L in infants and children. Three out of four infants in this study had serum 25(OH)D levels <50 nmol/L by the first month of life. A recent US study also found that 24 (73%) of 33 fully breastfeeding infants had serum 25(OH)D levels <50 nmol/L at one month of age (Wagner et al. 2010). In another study from India, ninety percent of breastfed infants <6 months old were reported to have serum 25(OH)D levels <50 nmol/L (Seth et al. 2009). Therefore, vit D deficiency (as defined by serum 25(OH)D <50 nmol/L) occurs early in breastfed infants and appears to be a public health problem. This should be of concern in view of the promotion of exclusive breast feeding and the additional recognition of increased risk of non-skeletal complications of vit D deficiency in children (Hypponen et al. 2001, Wayse et al. 2004, McNally et al. 2009).
We also showed that 18% and 12% of breastfed infants at the age of 4 and 26 weeks respectively had severe vit D deficiency which is consistent with higher risk of rickets (Institute of Medicine 2011, Dawodu et al. 2005). Infants born to African American mothers were 13 times much more likely than other infants to be severely vit D deficient soon after birth. In a recent study from Iowa, 5 (6%) of 77 light skin infants and 3 (43%) of 7 black skin pigmented infants respectively had severe vit D deficiency at 9 months of age (Ziegler et al. 2006). These findings are in agreement with the clinical experience of high prevalence of rickets among breastfed black infants in the US (Weisberg et al. 2004). In India (Seth et al. 2009), Greece (Challa et al. 2005) and the United Arab Emirates (Dawodu et al. 2003), 43%, 21–30% and 82% respectively of exclusively breastfed infants <6 months old were reported to be severely vit D deficient. Taken together, these results indicate that vit D deficiency among exclusively breastfed infants appears to be a global health problem and the cases presenting as rickets vastly underestimate the magnitude of vit D deficiency.
The negative correlation between 25(OH)D and PTH levels is supportive of suboptimal vit D status. However, significantly higher PTH was only demonstrated when serum 25(OH)D levels were <25 nmol/L in infants. Serum ALP was not associated with vit D deficiency in the infants at 4 weeks of age and during follow-up at 26 and 52 weeks. In contrast, a recent study found an association between elevated ALP and severe vit D deficiency (serum 25(OH)D <12.5 nmol/L) during the first 9 months of life (Ziegler et al. 2006). In this study we could not evaluate the relationship between ALP and severe vit D deficiency as defined by serum 25(OH)D <12.5 nmol/L because there were only 4 patients with serum 25(OH)D of 12.5 nmol/L and none with values <12.5 nmol/L. The results would suggest that significantly elevated serum PTH and ALP levels in infancy may only be associated with very low vit D status. This premise needs to be investigated in future large studies.
Vitamin D deficiency in infants at 4 weeks of age was associated with maternal vit D deficiency. The finding agrees with the results of other studies (Challa et al. 2005, Dawodu et al. 2003, Seth et al. 2009, Specker et al. 1985b) and indicate a possible contribution of maternal vit D deficiency to vit D deficiency in breastfed infants. Theoretically, vit D deficient lactating mothers would be expected to have been vit D deficient during pregnancy, although the current study did not evaluate vit D status of mothers before delivery. Maternal vit D deficiency during pregnancy is a significant risk factor for vit D deficiency in infants at birth (Merewood et al. 2010, Lee et al. 2007). Therefore, the early postnatal high rate of vit D deficiency likely results in part from poor previous vit D transplacental transfer (Hollis & Wagner 2004, Merewood et al. 2010, Lee et al. 2007) and low human milk vit D content (Wagner et al. 2006, Specker et al. 1985a).
Currently, the AAP recommends vit D supplementation soon after birth to prevent vit D deficiency in breastfed infants. It is, therefore, disappointing that only 5% of infants were reported to have received a vit D supplement by 4 weeks of age and only 19% in the first 6 months, despite the fact that the majority (68%) were still fully breastfed at 6 months. This low supplementation rate is consistent with the findings of 5–19% vit D supplementation rates among fully breastfed infants in other US studies (Perrine et al. 2010, Taylor et al. 2010, Davenport et al. 2004). Why then are breastfed infants not receiving vit D supplementation as recommended by recent publications from the AAP (Wagner & Greer 2008, Gartner & Greer 2003)? Previous studies that attempted to answer this question concluded that a majority of pediatric healthcare providers do not recommend vit D supplementation for breastfed infants (Davenport et al. 2004, Shaikh & Alpert 2004, Taylor et al. 2010). The reasons include the assumption that breast milk contains sufficient vit D (Sherman & Svec 2009, Davenport et al. 2004, Shaikh & Alpert 2004), infants receive adequate sunlight exposure (Sherman & Svec 2009, Shaikh & Alpert 2004), recommendation of supplementation might discourage mothers from breast feeding (Taylor et al. 2010) and that rickets is rare (Shaikh & Alpert 2004). On the contrary, rickets is not rare, especially among unsupplemented breastfed black infants in the US (Weisberg et al. 2004). Although assessment of vit D content of human milk was not conducted, most of the breastfeeding mothers in this study would be expected to produce milk with insufficient vit D for their nursing infants because of the high prevalence of maternal vit D insufficiency (Wagner et al. 2006). It is hoped that the results of this and other recent studies (Ziegler et al. 2006, Wagner et al. 2010) will alert pediatric health care providers to the magnitude of vit D deficiency and insufficiency in breastfeeding mothers and their infants and increase the awareness of the need to counsel for vit D supplementation of breastfeeding mother and her infant. Adequate supplementation should be emphasized, not only to prevent rickets but because of the reported association between vit D deficiency and increased risk of other childhood complications such as respiratory infections, wheezing and type 1 diabetes (Holick 2007, Hypponen et al. 2001, Wayse et al. 2004, McNally et al. 2009).
Contrary to the AAP recommendations to restrict sun exposure (AAP 1999), more than 90% of breastfed infants <6 months old in this study were reported by mothers to be exposed to direct sunlight and there was a positive correlation between skin sun exposure and vit D status of the infants. An older study of breastfed infants from Cincinnati (Specker et al. 1985b) also found a positive correlation between serum 25(OH)D and sun exposure score (minutes spent outside weighted by BSA exposed while outside). The authors concluded that vit D synthesis from skin exposure to sunlight contributes to the vit D status in largely unsupplemented exclusively breastfed infants. In this study of breastfeeding infants with very low rate of vit D supplementation, the changes in the serum 25(OH)D levels at follow-up reflected the seasonal changes in sunlight exposure. Higher serum 25(OH)D levels at 26 weeks in infants born in winter/spring and who reached 26 weeks during summer/fall is consistent with a higher cumulative endogenous synthesis of vit D due to higher sunlight skin exposures in summer/fall than winter/spring seasons. Weekly hours of sun exposure correlated positively with vit D status of infants during the first year. In addition to sun exposure, the higher vit D status during the follow-up possibly reflected an increase dietary intake of vit D because infants who received breast milk and fortified formula had higher serum 25(OH)D levels than those on breast milk only.
It is of interest that the median sun exposure of 3.8 h week-1 at 4 weeks of age in this study is similar to the average duration of sun exposure time of 4 h week-1 in another study over two decades ago from Cincinnati (Greer et al. 1982). These findings may indicate a lack of change in sun exposure behaviors of breastfed infants and that sun exposure still contributes to vit D status of breastfed infants during the first year of life despite the recommendation to limit sun exposure in infants. Since mothers still expose infants to direct sunlight, compliance with the AAP recommendation to avoid direct sun exposure (AAP 1999) could theoretically reduce endogenous vit D3 synthesis and contribute to higher prevalence of vit D deficiency in infants without corrective vit D supplementation. Therefore, it is important to quantify the contribution of sun exposure when evaluating vit D requirements in breastfed infants in future studies. Another important future research study is to investigate if there is a minimal-risk UVB skin exposure relative to skin cancer that promotes adequate vit D endogenous synthesis (Institute of Medicine 2011).
A significant finding in this study is the high prevalence of maternal vit D deficiency despite reported median daily intake of 540 IU of vit D, which is above the recommended daily intake of 200–400 IU vit D at the time of the study (Holick 2007, Hollis & Wagner 2004). This finding is in agreement with a recent study from Boston, Massachusetts, which showed that 35% of women who took daily prenatal vitamin containing 400 IU of vitamin D were vit D deficient at delivery (Merewood et al. 2010). In another study from Boston of mostly black mothers, 50% had serum 25(OH)D <30 nmol/L at delivery despite the majority were taking daily 400–600 IU vit D (Lee et al. 2007). At 4 weeks postpartum, 17% of the mothers in this cohort were vit D deficient and 66% were insufficient. This is not surprising because this amount of vit D supplementation is known to be inadequate to prevent vit D deficiency in pregnant and lactating mothers (Hollis & Wagner 2004, Wagner et al. 2006, Merewood et al. 2010, Lee et al. 2007). In addition, the majority (95%) of mothers with data on the use of sunscreen, reportedly used sunscreen with >8 SPF, which blocks endogenous synthesis of vit D3 by >95% (Matsuoka et al. 1987) and could have masked the effect of sun exposure on maternal vit D status. It is possible that inadequate endogenous vit D synthesis and a lack of corrective vit D intake likely contributed to the high prevalence of vit D insufficiency in the mothers, although relationship between sunscreen use and 25(OH)D could not be confirmed in a small sample of mothers with information on sun screen use in this study. Inadequate sun exposure and vit D intake were proposed in breastfeeding mothers in other high risk populations (Bhalala et al. 2007, Dawodu et al. 2003, Seth et al. 2009). In a setting of breastfeeding, vit D deficiency is better viewed as a mother-infant health problem. We suggest that global preventive strategies should ensure mother-infant vit D sufficiency and should start by ensuring vit D sufficiency during pregnancy.
The limitations of this study include a moderate sample size and population may not be representative of all communities. However, we examined these as potential bias, and found that the composition of ethnic groups in the study was similar to the distribution in Hamilton County where the study subjects were enrolled. In addition, 28% of the infants did not have serum 25(OH)D measured at 4 weeks of age because blood samples were not available. However, infants with serum 25(OH)D levels were representative of the study population because there were no significant differences in ethnicity, sun exposure behaviors, vit D supplementation and maternal vit D status between mother-infant pairs with and without 4 week infant serum 25(OH)D values (results not shown). The assessment of sun exposure behavior and infant vit D supplementation was based on reports by the mothers and are subject to recall bias; however, prospective collection of this information using 24-hour recalls may minimize this bias. In addition, assessment of sun exposure did not include data on time of day and the proportion of the body area being assessed that was actually exposed to direct sunlight. These factors affect endogenous vit D synthesis (Holick 2007). Therefore, caution is necessary in determining the magnitude of direct skin sun exposure and the rate of vit D supplementation use. Nevertheless, the study provides new information on sun exposure behaviors, and vit D supplementation of breastfeeding mothers and infants, type of infant milk feeding and the contribution of these factors to vit D status of breastfed infants during the first year of life.
Conclusions
Vitamin D deficiency is widespread early postpartum in breastfeeding mothers and their infants in Cincinnati, and severe vit D deficiency is common in the infants, especially African Americans. Winter/spring seasons, low skin sun exposure and longer duration of exclusive breastfeeding are independent determinants of vit D deficiency in breastfeeding infants with low rate of reported vit D supplementation during the first year of life. We suggest that there is a need to intensify corrective vit D supplementation starting during pregnancy to prevent vit D deficiency in breastfeeding mothers and infants when sunlight exposure is purposely avoided or restricted.
Key Messages.
Vitamin D (vit D) deficiency is widespread and detectable soon after birth in breastfeeding mothers and their infants in Cincinnati.
Maternal compliance with recommended infant vit D supplementation is poor.
Infant exposure to sunshine exceeds AAP recommendations. At 26 and 52 weeks of age, season of birth and weekly hours of sun exposure are major determinants of vit D status.
We suggest intensified vit D supplementation strategy that ensures vit D sufficiency during pregnancy and in breastfeeding mothers and infants, especially, when sunlight exposure is limited or restricted.
Acknowledgments
The authors thank Suzanne Summer, RD for the assessment of dietary vitamin D intake of each subject from the dietary recall data.
Sources of Funding
This study was supported in part by Mead Johnson Nutrition, Inc., Institutional Clinical and Translational Science Award, and NIH HD13021.
Abbreviations
- vit D
Vitamin D
- AAP
American Academy of Pediatrics
- PTH
Parathyroid Hormone
- 25(OH)D
25-hydroxyvitamin D
- BSA
Body surface area
- SPF
Sun protection factor
- ALP
Alkaline Phosphatase
Footnotes
Financial Disclosure: No financial relationship to disclose.
Conflict of interest
The authors declare no conflicts of interest.
Contributions
AD, JW and AM participated in the study design, data analysis and the initial draft of the manuscript. JW PH and AM provided statistical guidance in the analysis. AD, JW, AM, PH, LZ, BD and JH assisted in interpretations of results. All the co-authors contributed to the preparation and review of the content of the manuscript.
References
- AAP. Ultraviolet light: a hazard to children. Committee on Environmental Health. Pediatrics. 1999;104:328–33. [PubMed] [Google Scholar]
- Andiran N, Yordam N, Ozon A. Risk factors for vitamin D deficiency in breast-fed newborns and their mothers. Nutrition. 2002;18:47–50. doi: 10.1016/s0899-9007(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87:4952–6. doi: 10.1210/jc.2002-020636. [DOI] [PubMed] [Google Scholar]
- Bhalala U, Desai M, Parekh P, Mokal R, Chheda B. Subclinical hypovitaminosis D among exclusively breastfed young infants. Indian Pediatr. 2007;44:897–901. [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- Challa A, Ntourntoufi A, Cholevas V, Bitsori M, Galanakis E, Andronikou S. Breastfeeding and vitamin D status in Greece during the first 6 months of life. Eur J Pediatr. 2005;164:724–9. doi: 10.1007/s00431-005-1757-1. [DOI] [PubMed] [Google Scholar]
- Davenport ML, Uckun A, Calikoglu AS. Pediatrician patterns of prescribing vitamin supplementation for infants: do they contribute to rickets? Pediatrics. 2004;113:179–80. doi: 10.1542/peds.113.1.179. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Absood G, Patel M, Agarwal M, Ezimokhai M, Abdulrazzaq Y, et al. Biosocial factors affecting vitamin D status of women of childbearing age in the United Arab Emirates. J Biosoc Sci. 1998;30:431–7. doi: 10.1017/s0021932098004313. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142:169–73. doi: 10.1067/mpd.2003.63. [DOI] [PubMed] [Google Scholar]
- Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher prevalence of vitamin D deficiency in mothers of rachitic than nonrachitic children. J Pediatr. 2005;147:109–11. doi: 10.1016/j.jpeds.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Gartner LM, Greer FR. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111:908–10. doi: 10.1542/peds.111.4.908. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162:505–12. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen-Asche PS, Tsang RC. Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. J Pediatr. 1982;100:919–22. doi: 10.1016/s0022-3476(82)80514-3. [DOI] [PubMed] [Google Scholar]
- Ho ML, Yen HC, Tsang RC, Specker BL, Chen XC, Nichols BL. Randomized study of sunshine exposure and serum 25-OHD in breast-fed infants in Beijing, China. J Pediatr. 1985;107:928–31. doi: 10.1016/s0022-3476(85)80192-x. [DOI] [PubMed] [Google Scholar]
- Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–26. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–4. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- Liang L, Chantry C, Styne DM, Stephensen CB. Prevalence and risk factors for vitamin D deficiency among healthy infants and young children in Sacramento, California. Eur J Pediatr. 2010;169:1337–44. doi: 10.1007/s00431-010-1226-3. [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–8. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981–8. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- Merewood A, Mehta SD, Grossman X, Chen TC, Mathieu JS, Holick MF, et al. Widespread vitamin D deficiency in urban Massachusetts newborns and their mothers. Pediatrics. 2010;125:640–7. doi: 10.1542/peds.2009-2158. [DOI] [PubMed] [Google Scholar]
- Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125:627–32. doi: 10.1542/peds.2009-2571. [DOI] [PubMed] [Google Scholar]
- Seth A, Marwaha RK, Singla B, Aneja S, Mehrotra P, Sastry A, et al. Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22:241–6. doi: 10.1515/jpem.2009.22.3.241. [DOI] [PubMed] [Google Scholar]
- Shaikh U, Alpert PT. Practices of vitamin D recommendation in Las Vegas, Nevada. J Hum Lact. 2004;20:56–61. doi: 10.1177/0890334403260617. [DOI] [PubMed] [Google Scholar]
- Sherman EM, Svec RV. Barriers to vitamin D supplementation among military physicians. Mil Med. 2009;174:302–7. doi: 10.7205/milmed-d-01-4708. [DOI] [PubMed] [Google Scholar]
- Specker BL, Tsang RC. Cyclical serum 25-hydroxyvitamin D concentrations paralleling sunshine exposure in exclusively breast-fed infants. J Pediatr. 1987;110:744–7. doi: 10.1016/s0022-3476(87)80016-1. [DOI] [PubMed] [Google Scholar]
- Specker BL, Tsang RC, Hollis BW. Effect of race and diet on human-milk vitamin D and 25-hydroxyvitamin D. Am J Dis Child. 1985a;139:1134–7. doi: 10.1001/archpedi.1985.02140130072032. [DOI] [PubMed] [Google Scholar]
- Specker BL, Valanis B, Hertzberg V, Edwards N, Tsang RC. Sunshine exposure and serum 25-hydroxyvitamin D concentrations in exclusively breast-fed infants. J Pediatr. 1985b;107:372–6. doi: 10.1016/s0022-3476(85)80509-6. [DOI] [PubMed] [Google Scholar]
- Strand MA, Perry J, Jin M, Tracer DP, Fischer PR, Zhang P, et al. Diagnosis of rickets and reassessment of prevalence among rural children in northern China. Pediatr Int. 2007;49:202–9. doi: 10.1111/j.1442-200X.2007.02343.x. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin d among infants breastfed for prolonged periods. Pediatrics. 2010;125:105–11. doi: 10.1542/peds.2009-1195. [DOI] [PubMed] [Google Scholar]
- Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16. doi: 10.1179/146532806X90556. [DOI] [PubMed] [Google Scholar]
- Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- Wagner CL, Howard C, Hulsey TC, Lawrence RA, Taylor SN, Will H, et al. Circulating 25-hydroxyvitamin d levels in fully breastfed infants on oral vitamin d supplementation. Int J Endocrinol. 2010:235035. doi: 10.1155/2010/235035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1:59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004;80:1697S–705S. doi: 10.1093/ajcn/80.6.1697S. [DOI] [PubMed] [Google Scholar]
- Zeghoud F, Vervel C, Guillozo H, Walrant-Debray O, Boutignon H, Garabedian M. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. Am J Clin Nutr. 1997;65:771–8. doi: 10.1093/ajcn/65.3.771. [DOI] [PubMed] [Google Scholar]
- Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics. 2006;118:603–10. doi: 10.1542/peds.2006-0108. [DOI] [PubMed] [Google Scholar]