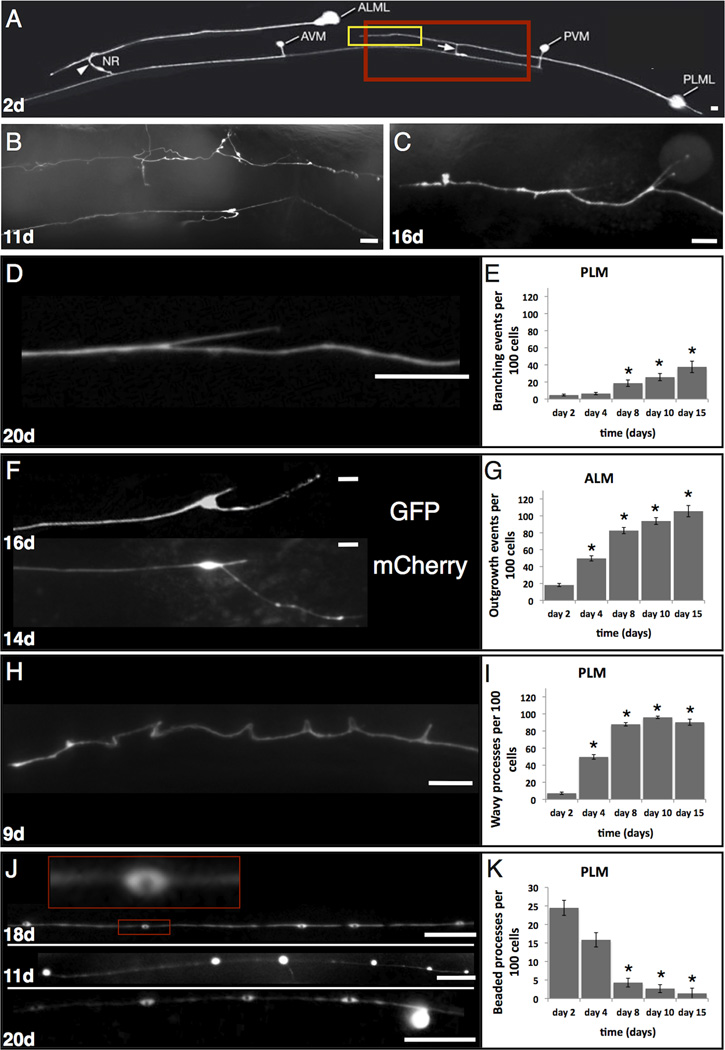
Figure 1. C. elegans touch receptor neurons exhibit morphological changes with age.
A. GFP-visualized touch receptor neurons in a 2 day old adult. Strain ZB154 zdIs5[pmec-4GFP] houses an integrated transgene expressed in six touch receptor neurons, only the left anterior ALM and posterior PLM are visible in this focal plane. Native branches at distal ends of ALM (arrowhead) and PLM (arrow) are indicated. Highlighted boxes indicate the regions analogous to those magnified in panel B. Note the uniform distribution of GFP along the processes and the short posterior extension from the PLM soma typical for young adults. Panel is taken from www.wormatlas.org. Scale bars represent 10 microns in all panels.
B–C. Examples of dramatic morphological changes that can occur in aging touch neurons.
B. Magnified posterior mid body section (~corresponding to red box in A) of an 11 day old animal (25°C), strain zdIs5[pmec-4GFP]; PLMR is the top neuron, PVM is the bottom neuron.
C. Magnified body section (~corresponding to yellow box in A) in a 16 day old PLML (25°C), strain zdIs5[pmec-4GFP]. Neuronal processes exhibit novel branches, wavy appearance, and some GFP-beading.
D–I. Examples and quantitation of morphological changes in aging touch receptor neurons. For all experiments, day 1 is the beginning of adulthood (24°C) and animals were scored only on the day indicated, rather than sequentially. Numbers of neurons of indicated types scored per time point were: 428 day 2; 340 day 4; 280 day 8; 224 day 10; 72 day 15. Unless otherwise stated, photographs are of strain ZB154 zdIs5[pmec-4GFP], anterior is to the left.
D. Typical branched process phenotype in an aging PLM neuron, 20 days old.
E. Quantitation of novel dendrite branching phenotypes in PLM neurons in aging adults. Total numbers of major novel branches observed/100 neurons are indicated. We counted branches across the entire anteriorly projecting process, excluding the normal developmental branch. We scored at 600X magnification using a dissecting microscope for relatively high throughput scoring up to day 10. Small novel branches would have been missed at this magnification, so the rate of branch occurrence presented is a likely underestimate. At day 15, gut autofluorescence often precluded observation of branching in the dissecting microscope, so we scored at 600X on a compound scope with a higher numerical aperture objective (10x) to limit background fluorescence. Tiny novel branches visualized with the compound microscope at day 15 were not included in the statistical analysis, so 15 day data count only large branch scores similar to those detected with the dissecting microscope. Asterisk indicates p<0.05 for independent T test, comparison to day 2 adult, error bars indicate the standard deviation.
F. Typical soma outgrowth phenotypes in ALM neurons. Top, zdIs5[pmec-4GFP], 16 day old; bottom, strain KWN177, rnyIs014[pmec-4mCherry; unc-119(+)], 14 days old.
G. Quantitation of soma outgrowths in aging ALM neurons. In young adults, we find only ~18% of soma exhibit the small posterior extension in 2 day old adults. Because of the relatively infrequent occurrence of this feature, we counted the presence of any extension as one outgrowth event. Total numbers of outgrowths per 100 cell observations are indicated. Some soma-anchored processes can grow substantially with increasing age (although growth appears to stop several days prior to death), but we have not quantitatively scored the total amount of growth. Microscopy protocol for observation was as described for panel E; statistics as in panel E.
H. Example of a PLM neuron of zdIs5[pmec-4GFP] in which the process appears wavy and exhibits some acute bends, 9 days old.
I. Quantitation of wavy process occurrence in aging PLM neurons. We scored a process as wavy if it appeared wavy or bent at any place along the process (in contrast to the normal straight trajectory); waves or bends remained in place in a moving animal, so appear to be fixed characteristics of the neuron rather than dependent upon the posture of the animal. Scoring microscopy was as described for panel D. Error bars indicate standard deviation, statistics as in panel 1E.
J. Examples of bead-like GFP accumulations in PLM processes. Top, GFP accumulates with a beaded appearance with central regions of GFP exclusion—inset, enlarged view of a “bead” with a dark, GFP-free center (18 days old). Middle, uniform bright accumulations that sometimes appear to extend off the main process as protrusions (11 days old). Bottom, a process with both beaded structures and a rounded extension protruding from the right end of the process (20 days old). Beads are unlikely to correspond to novel synaptic structures on the normally synapse-free sensory process as the synaptic protein reporter SNB-1::GFP does not concentrate at these beads to levels observed at synapses and this SNB-1::GFP faint signal can still be observed in the absence of beads/protrusions; faint SNB-1::GFP signals can be found in about 1/3 of transient beads that move as if being trafficked (data not shown). Other work has shown that similar beads/protrusions can include disorganized microtubules and do not correlate with a lysosomal marker (Pan et al., 2011).
K. Quantitation of the occurrence of beaded processes of aging PLM neurons. We scored a process as containing GFP beads or protrusions if there were more than 10 GFP accumulations per process; both uniformly filled and central GFP exclusion beads were counted together. Microscopy scoring was as described for panel D. Error bars indicate standard deviation, statistics as in panel 1E.