Abstract
Upon antigen stimulation, naïve T helper cells differentiate into distinct lineages to attain specialized properties and effector functions. TH17 cells, a recently identified lineage of CD4+ effector T cells, play a key role in the immune defense against fungi and extracellular bacteria but also contribute to the pathogenesis of many autoimmune conditions. The differentiation of TH17 cells is orchestrated by an intricate network of signaling pathways and transcriptional regulators in T cells. While the involvement of T cell-intrinsic pathways has been described extensively, we are just beginning to appreciate how TH17 cell development is shaped by extrinsic pathways especially the innate immune signals. Dendritic cells (DCs), the most important cell type to bridge innate and adaptive immunity, drive TH17 cell differentiation by providing antigenic, costimulatory and cytokine signals. This is mediated by the recognition of innate and inflammatory signals by DCs via pattern recognition receptors, cytokine receptors and other immunomodulatory receptors that in turn activate the intracellular signaling network. In particular, p38α MAP kinase has emerged as a critical pathway to program DC-dependent TH17 cell differentiation by integrating multiple instructive signals in DCs. Here we summarize the current knowledge on the mechanisms by which DC-derived innate immune signals drive TH17 cell differentiation.
Keywords: dendritic cells, innate immunity, MAPK, T cell differentiation, TH17 cells
INTRODUCTION
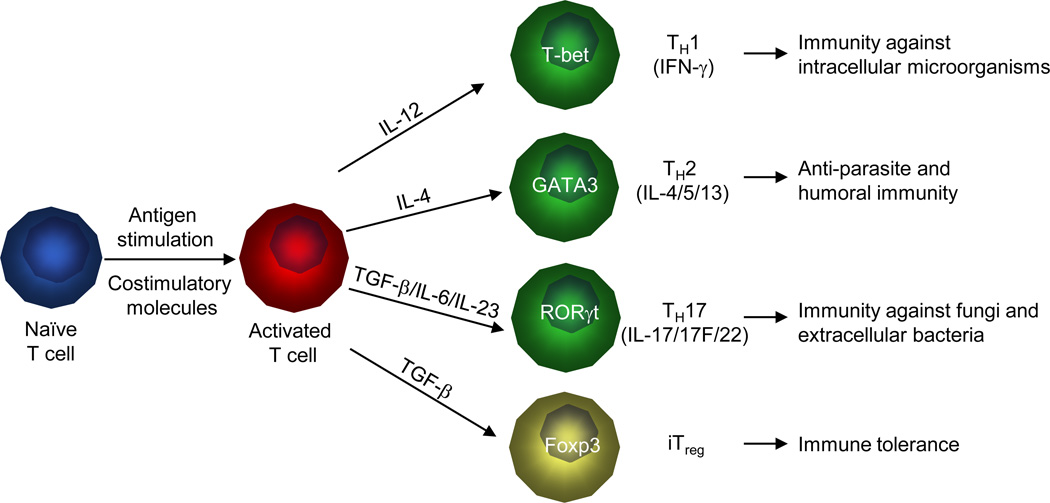
For more than twenty years, it has been appreciated that naïve CD4+ T cells can differentiate into distinct lineages to attain specialized properties and effector functions. For the initially identified T cell subsets, TH1 cells are characterized by high production of IFN-γ and are necessary to clear intracellular pathogens. TH2 cells produce the signature cytokine interleukin-4 (IL-4) and are effective at controlling helminthes1. A new subset of IL-17-producing T (TH17) cells has recently been described to mediate immune defense against fungi and extracellular bacteria and tissue inflammation in autoimmune diseases. TH17 cell differentiation can be initiated by transforming growth factor-beta (TGF-β) in the presence of inflammatory cytokines IL-6 or IL-21, and is further reinforced by IL-23. TH17 cells produce several signature cytokines including IL-17, IL-17F and IL-22, which provoke inflammatory responses including neutrophilia, tissue remodeling, and production of antimicrobial proteins2-5. In the absence of the proinflammatory inputs, TGF-β drives naïve CD4+ T cells to develop into induced Foxp3+ regulatory T (iTreg) cells, which act in synergy with natural Treg (nTreg) cells to promote immune tolerance and inhibit autoimmunity5. Although the presence of additional T cell subsets and the plasticity of T cell lineage choices have been appreciated, these four T cell populations represent the widely accepted major T cell lineages (Figure 1). The specification of T cell lineages is orchestrated by an intricate network of signaling pathways and transcriptional master regulators in T cells. While the involvement of T cell-intrinsic pathways has been described extensively, how T cell differentiation is triggered by extrinsic pathways and physiological stimuli is relatively less understood.
Figure 1. T cell lineage commitment and function.
Upon encountering foreign antigens and costimulatory molecules presented by DCs, naïve CD4+ T cells can differentiate into TH1, TH2, TH17 and iTreg cells. These differentiation programs are mainly shaped by cytokines produced by DCs and are characterized by the expression of lineage-specific transcription factors and production of signature cytokines. IL-12 is important for the differentiation of TH1 cells with an important function in host defense against intracellular pathogens. In response to IL-4, naïve T cells differentiate into TH2 cells, which play a crucial role in anti-parasite and humoral immunity. TGF-β together with IL-6 and IL-23 instruct naïve T cells to develop into TH17 cells that mediate immunity against fungi and extracellular bacteria. In the absence of inflammatory cytokines, TGF-β promotes naïve T cells to differentiate into Foxp3-expressing iTreg cells for the maintenance of immune tolerance.
Antigen-presenting cells (APCs) drive T cell differentiation by providing the antigenic, costimulatory and cytokine signals. DCs are the most important APCs to bridge the crosstalk between innate and adaptive immunity6, 7. DCs express a repertoire of pattern recognition receptors (PRRs) that sense microbial pathogen products and endogenous ligands to initiate a signaling cascade culminating in the activation of DCs and induction of adaptive immunity. Activated DCs present high levels of major histocompatibility complex (MHC) molecules bearing pathogen-derived peptides, which engage T cell receptors (TCRs) on naïve antigen-specific T cells. This delivers the first activating signal to the T cell and is therefore referred to as ‘signal 1’. DCs activated by pathogen encounter also upregulate costimulatory molecules to bind counter-receptors on T cells and transmit signals that are important for T cell proliferation and survival (signal 2). Finally, activated DCs also produce cytokine and non-cytokine mediators that act on the T cell to promote its differentiation into an effector cell (signal 3). The integration of these three classes of signals by the responding T cells, to a large extent, determines their subsequent fate.
In this review, we summarize the recent findings on the mechanisms by which DC-mediated innate immune signals drive TH17 cell differentiation and inflammation. We first provide a brief overview of intrinsic pathways, especially transcriptional mechanisms, that specify the TH17 lineage choice. We then discuss how DC-derived immune signals, especially the polarizing cytokines, mediate TH17 cell differentiation by shaping the interface between DCs and T cells. We next describe how receptor-specific signals instruct DCs to drive TH17 cell differentiation, by focusing on the roles of PRRs and other immunomodulatory receptors expressed by DCs. Finally, we highlight the emerging data that the stress-activated p38 MAP kinase (MAPK) pathway integrates diverse instructive signals in DCs for TH17 cell differentiation.
INTRINSIC CONTROL OF TH17 CELL DIFFERENTIATION: AN OVERVIEW
Since the seminal discovery of TH17 cells as a separate lineage8, 9, multiple transcription factors have been identified as critical regulators of TH17 cell differentiation10. Among the lineage-restricted transcription factors essential for TH17 cell differentiation are two retinoic acid-related organ receptors, RORγt and RORα11, 12. Similar to T-bet in TH1 cells and GATA3 in TH2 cells, RORγt and RORα are highly induced in TH17 cells and play central roles in specifying the lineage differentiation. Overexpression of either transcription factor promotes TH17 cell differentiation when TH1 and TH2 differentiation is blocked. Deficiency of RORγt, and to a considerably lesser extent, of RORα, impairs TH17 cell differentiation, whereas loss of both factors completely inhibits TH17 cell differentiation and suppresses the development of experimental autoimmune encephalitis (EAE), a TH17-mediated autoimmune disease11, 12. Thus, RORγt and RORα have redundant functions, with RORγt playing a more dominant role in specifying the TH17 cell fate.
Induction of RORγt and RORα under TH17-polarizing conditions is dependent upon STAT3, which perceives and transduces signals from IL-6, IL-23 and other cytokines. Deficiency of STAT3 decreases the expression of lineage-specific transcription factors and the TH17 family cytokines, whereas enhancing STAT3 activity via deletion of the negative regulator SOCS3 increases IL-17 expression13-15. Aside from RORγt, RORα and STAT3, additional transcriptional factors contribute to TH17 cell differentiation and/or cytokine expression. These include IRF416, BATF17, RUNX118, and c-Maf19, most of which have additional roles in other aspects of T cell lineage choices in the periphery or thymus. Finally, TH17 cell differentiation is further shaped by transcription factors with prominent roles in environmental sensing. Two of the most notable examples are AHR and HIF1α, which sense environmental toxins and hypoxic conditions, respectively20–23. Therefore, differentiation of TH17 cells requires coordinated actions of multiple transcription factors. These transcriptional mechanisms act in synergy with the signaling networks, metabolic pathways and epigenetic regulators to perceive and transduce diverse lineage specification signals derived from DCs.
IMMUNE SIGNALS AT THE DC–T CELL INTERFACE FOR TH17 POLARIZATION
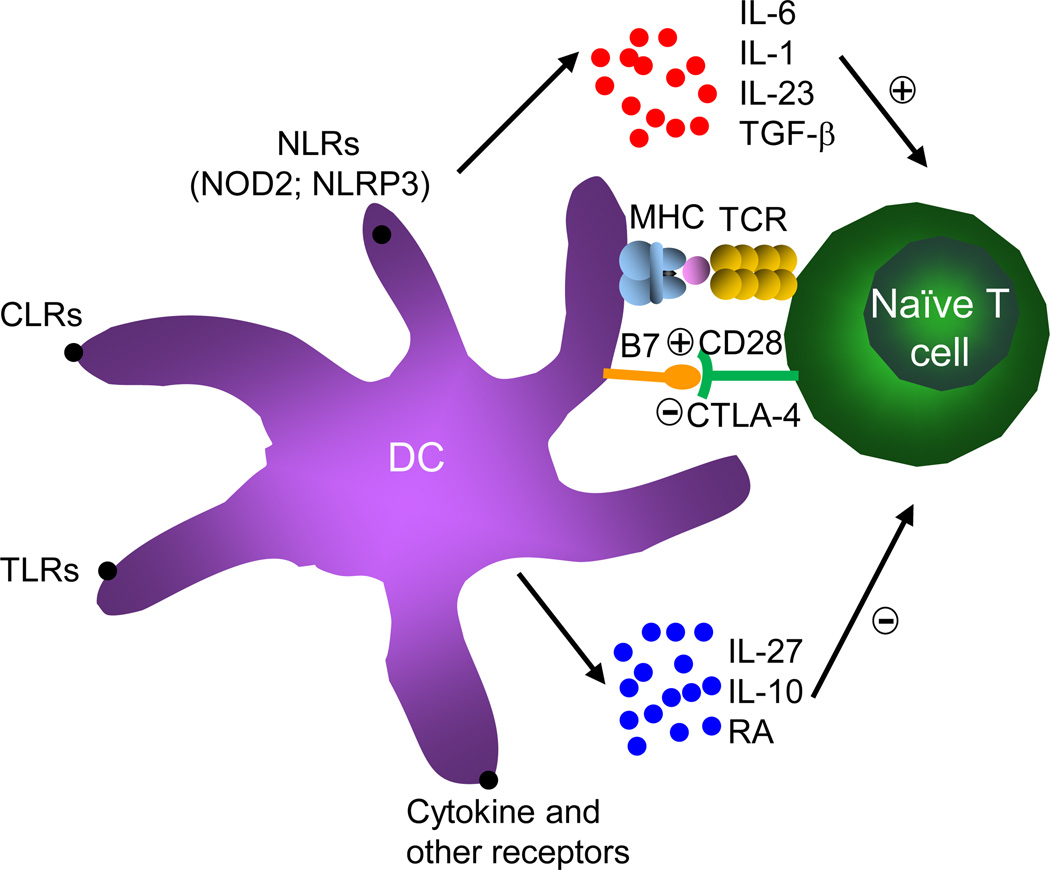
DCs are the most potent cell type to deliver antigens, costimulation and cytokines to T cells for their proper activation and differentiation. As compared with the development of other T cell lineages, differentiation of TH17 cells has selective requirements for DC-derived signals. The integration of these external signals by T cells ultimately dictates the quality and quantity of TH17-mediated immune responses (Figure 2).
Figure 2. DC-derived innate signals instruct TH17 cell differentiation.
DCs sense pathogens or other stimuli via various cell surface receptors, including TLRs, CLRs, NLRs, cytokine receptors and other immunomodulatory receptors. DCs activated by these stimuli then present MHC bearing antigen-specific peptides to engage TCRs on naïve T cells. Upon stimulation, DCs also upregulate costimulatory molecules B7 to bind the corresponding receptors (CD28 or CTLA-4) expressed by T cells. Moreover, activated DCs produce positive (red color) or negative (blue color) TH17-polarizing cytokines and non-cytokine mediators to regulate TH17 cell differentiation.
Antigenic signals
It has been known for some time that fate determination of T cells is shaped by the strength of TCR signals. Specifically, high antigen doses promote the generation of TH1 cells, whereas low doses of the same antigen favor TH2 cell polarization24, 25. The effects of antigen doses were later found to correlate with the extent of CD40L upregulation on T cells26. Iezzi et al. recently reported that the strength of antigenic stimulation also critically influences TH17 cell differentiation, because high, but not low or intermediate, antigen concentrations favor IL-17 production27. Strong antigenic stimulation of T cells upregulates CD40L expression, which acts in concert with certain microbial stimuli to enhance IL-6 production from DCs to drive TH17 polarization. Compared with TH1 cells, TH17 cells appear to require even stronger antigen stimulation for their development, and this is associated with profound upregulation of CD40L expression on T cells that in turn delivers CD40L–CD40-mediated costimulation to DCs27. Indeed, CD40 deficiency diminishes IL-6 production from DCs and markedly reduces TH17 responses in models of EAE and stimulation with the Gram-positive bacterium Propionibacterium acnes27, 28. We also observed defective TH17 cell differentiation mediated by CD40-deficient DCs in vitro, suggesting the involvement of direct DC and T cell interaction29. Therefore, high antigen concentrations favor TH17 cell differentiation by fostering the CD40L–CD40 cross-talk at the DC–T cell interface.
Costimulatory molecules
DCs express a number of costimulatory molecules including CD80 (B7-1) and CD86 (B7-2) on their cell surface to engage the corresponding receptors, such as CD28, on T cells. This interaction transmits signals to promote T cell proliferation and survival. Park et al. reported that APCs deficient in both B7 molecules failed to instruct T cell differentiation into TH17 cells8. Odobasic et al. later demonstrated that inhibition of CD86, but not CD80, suppressed IL-17 production from splenocytes and decreased T cell accumulation in the joints in the model of antigen-induced arthritis30. Consistent with this, our recent study indicated that blocking CD86 function downregulated DC-dependent TH17 cell differentiation in vitro29. Conversely, CTLA4, a negative factor for T cell activation that also interacts with B7 molecules on DCs, inhibits TH17 cell differentiation in vitro and in vivo and suppresses TH17-mediated autoimmunity31. The inducible costimulatory ICOS is another member of the CD28 superfamily that also regulates naïve T cell activation. ICOS signaling in T cells is required for efficient TH17 development and expansion in both murine and human systems8, 32, indicating the therapeutic potentials of ICOS modulation for the treatments of TH17-dependent disorders. ICOS functions by inducing c-Maf and transactivating IL-21, an important T cell autocrine factor for TH17 cell differentiation19. These findings collectively indicate that TH17 cell differentiation requires selective costimulatory signals from DCs.
Polarizing cytokines
Among the most potent factors to polarize TH17 cell differentiation are STAT3-activating cytokines IL-6, IL-21 and IL-23, along with TGF-β and IL-1. IL-6, IL-23 and IL-1 are mainly produced by the innate immune system especially DCs, whereas IL-21 and TGF-β can be produced by T cells in an autocrine/paracrine manner to further shape TH17 cell development. Earlier studies showed that IL-17-producing cells could be efficiently generated in vitro from naïve CD4+ T cells activated with TCR and costimulation in the presence of IL-6 and TGF-β33–35. Although less effective than IL-6 in initiating TH17 cell differentiation, IL-21 produced by developing TH17 cells has an important role to propagate the differentiation process15, 36, 37. In contrast to IL-6, IL-23 does not act on naïve T cells, because the receptor for IL-23 is induced only in T cells after stimulation in the presence of IL-6 or IL-21. Therefore, IL-23 is more important in the later phase of TH17 cell differentiation and in the maintenance of the TH17 phenotype38. However, this function of IL-23 is indispensable for the pathogenicity of TH17 cells, as mice lacking IL-23 are completely resistant to EAE39. The roles of IL-23 signaling in the development of human TH17-related autoimmune diseases were further highlighted by recent findings showing the association of IL-23R polymorphisms and the prevalence of several autoimmune diseases40. Therefore, the three STAT3-activating cytokines IL-6, IL-21 and IL-23 are critically involved in the initiation, amplification and stabilization stages of TH17 cell differentiation, respectively. Another proinflammatory cytokine, IL-1, also pays a crucial role in early TH17 cell differentiation by signaling through IL-1R1 and the downstream Myd88–TRAF6 pathway to promote IRF4 and RORγt expression in T cells41, 42.
Compared with the well described roles of these proinflammatory cytokines in TH17 responses, whether and how the immunosuppressive cytokine TGF-β regulates TH17 cell differentiation remain incompletely understood. Addition of TGF-β to IL-6 or IL-21 enhances the development of TH17 cells in vitro15, 36, 37, and deletion of TGF-β1 specifically from T cells lowers TH17 cell generation and EAE43. Moreover, phagocytosis of infected apoptotic cells by DCs triggers the release of both IL-6 and TGF-β to instruct TH17 cell differentiation during infection44. However, high doses of TGF-β downregulate the expression of IL-23R, the key pathogenic molecule associated with TH17 cells, and instead provoke Foxp3 expression and iTreg induction45. Further, Ghoreschi et al. described the generation of TH17 cells that are mediated by IL-6, IL-23 and IL-1β in the complete absence of TGF-β signaling. Compared with TH17 cells derived from IL-6 and TGF-β, the TGF-β-independent TH17 cells exhibit distinct expression profiles and more importantly, stronger pathogenicity in the EAE model46. These results highlight the heterogeneity of TH17 responses and context-dependent effects of TGF-β on T cell fate decision. Equally complex is the source(s) of TGF-β to drive TH17 responses. Although TGF-β derived from Treg cells was initially thought to promote TH17 cell differentiation33, recent genetic studies highlighted an important role of TGF-β1 produced by activated T cells, but not Treg cells, to promote TH17 responses47. Moreover, DCs were also shown to be an important source of TGF-β for TH17 cell differentiation48. Consistently with this notion, expression of the integrin αvβ8, which is required to activate TGF-β, plays a critical role in the differentiation of TH17 cells49, 50. Additional studies are required to ascertain the precise function and regulation of TGF-β in TH17 cell differentiation.
TH17 cell differentiation is shaped by both positive and negative polarizing cytokines. IL-27 is arguably the most potent cytokine produced by DCs to limit TH17 cell differentiation and autoimmune inflammation. Mice deficient in IL-27R are hyper-susceptible to EAE and other inflammatory disorders and generate more TH17 cells51–53. Multiple mechanisms mediate the effects of IL-27 for the inhibition of TH17 cell development54. A particularly important mechanism is IL-27-dependent induction of IL-10-expressing Tr1 cells that play a central role in downregulating the proinflammatory TH17 cell responses55–57. Furthermore, IL-27 inhibits TH17 cell differentiation via directly inhibiting RORγt and RORα expression and promoting T-bet expression and TH1 generation54. In addition to IL-27, production of IL-10 by DCs has also been shown to inhibit TH17 cell differentiation via constraint of IL-1 production by DCs58. Several T cell-derived cytokines, such as IFN-γ, IL-4 and IL-2, have also been shown to inhibit TH17 cell development8, 9, 59, 60. Since DCs are not the major producer of these cytokines, such regulation is not further discussed here. The readers are encouraged to read excellent reviews on this topic2–5.
In summary, differentiation of TH17 cells is largely determined by the innate immune signals transduced from DCs and the integration of these signals by responding T cells. In particular, DC-derived cytokines deliver a critical ‘signal 3’ to T cells, to mediate both positive and negative regulation of TH17 cell differentiation. Aside from antigens, costimulation and cytokines, recent work has identified that differentiation of TH17 cells is influenced by additional immune modulators produced by DCs. For example, retinoic acid, a vitamin A metabolite produced by CD103+ DCs in the gut-associated lymphoid tissues (GALTs), inhibits TH17 cell differentiation while promoting iTreg generation by activating the retinoic acid receptor61–63. Moreover, while the crosstalk between DC and T cells plays a central role in programming TH17 cell differentiation, additional cell types modulate functions of DCs and T cells and/or the immune microenvironment to impinge upon TH17 cell differentiation. In this context, mice deficient for γδ T cells have markedly attenuated TH17-mediated autoimmune disease64. Mechanistically, IL-17 expressed by this lymphocyte subset activates IL-17R on epithelial and stromal cells, resulting in the production of IL-6, IL-1 and other inflammatory cytokines that act in a positive feedback loop on the γδ T cells and differentiating TH17 cells to amplify inflammation5, 64.
SENSING TH17-INSTRUCTIVE STIMULI BY DC RECEPTORS
In agreement with a potent proinflammatory function of TH17 cells, TH17-mediated responses are strongly induced in models of autoimmune and infectious diseases. van de Veerdonk et al. directly compared the effects of different pathogens to elicit TH17 responses, and found that the fungus Candida albicans was much more potent than various bacteria tested to induce IL-17 production from human PBMC65. We also found that heat-killed fungi had much greater adjuvant activity than bacteria to stimulate antigen-specific T cells to differentiate into TH17 effector cells in vivo29. Further, under steady-state conditions, a sizable population of IL-17+ CD4+ T cells is detectable in GALTs such as lamina propia from small intestine in response to commensal microbiota66. How do the diverse innate stimuli and microbial agents instruct TH17 responses? PRRs expressed by DCs, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs) and NOD-like receptors (NLRs), are essential to sense these TH17-instructive stimuli. Cytokine receptors and other immunomodulatory receptors also contribute to DC-mediated TH17 cell differentiation (Figure 2). Here we summarize the functions of these receptors, including mechanisms by which these receptors transduce signals via receptor-proximal downstream signaling.
TLRs
TLRs, the first group of PRRs identified, are classically considered to polarize toward TH1 responses67. Recent studies indicate that ligation of certain TLRs on DCs potentiates TH17 cell differentiation by affecting production of TH17-polarizing cytokines27, 68, 69. TLR-mediated effects on TH17 responses are generally much weaker than those induced by CLRs (see below for details)70, 71. Further, several TLRs, including TLR2, TLR6 and TLR7, have been implicated in negatively regulating TH17 responses in inflammatory and infectious models72–75. This is associated with the abilities of these receptors to downregulate IL-23 production from DCs. Therefore, TLR signaling in DCs can either positively or negatively regulate TH17 cell differentiation, in a receptor and stimulation-specific manner. It should also be noted that T cells themselves express TLRs and respond to TLR stimulation. T cells stimulated with TLR2 agonists exhibit enhanced TH17 cell differentiation, and loss of TLR2 in CD4+ T cells ameliorates EAE76. The cell type-specific roles of TLRs in TH17 cell differentiation require additional studies, which can be assisted with the generation and analysis of conditional alleles of these receptors.
CLRs
CLRs are a large family of proteins characterized by the presence of one or more C-type lectin-like domains (CTLDs). Several CLRs recognize carbohydrates in the cell wall of fungi and other pathogens (such as β-glucans) and signal through the intracellular kinase Syk to initiate inflammatory responses in both innate and adaptive immunity77. Ligation of these CLRs on DCs is an important mechanism by which fungal infection induces strong TH17 cell responses. LeibundGut-Landmann et al. reported that the prototypical CLR, Dectin-1 (also known as CLEC7a), activates Syk and the adaptor CARD9 to promote DC maturation and production of TH17-polarizing cytokines, thereby instructing T cell differentiation into the TH17 lineage. Infection with C. albicans induces CARD9-dependent TH17 responses and protective immunity70. Further studies showed that Dectin-2 (CleC6A), which also signals through Syk and CARD9, is even more potent than Dectin-1 to mediate TH17 cell responses and protection from C. albicans infection78, 79. Moreover, mannose receptor, another member of the CLR family, is also important to induce IL-17 production in response to C. albicans65. These results collectively highlight potent effects of CLRs in driving TH17 responses. Moreover, synergic interactions between CLRs and TLRs further modulate T cell fate79, 80.
NLRs
NLRs are a family of innate immune receptors for intracellular microbial sensing characterized by the presence of a conserved NOD domain81. NLRs are implicated in a multitude of innate immune signaling pathways ranging from the regulation of MAPK and NF-κB signaling pathways by NOD1 and NOD2, to the assembly of caspase-1-activating protein complexes named ‘inflammasomes’ by the NLR protein NLRP381. Recent studies have identified important roles for NLRs to bridge innate and adaptive immunity by promoting TH17 cell differentiation. NOD1 and NOD2, members of the NLR family, detect muramyldipeptide (MDP), a derivative of bacterial peptidoglycan and signal through downstream kinase RIP2. Activation of NOD2 synergizes with TLR ligation to induce DC production of IL-23 and IL-1 that drive TH17 cell responses in human memory T cells. NOD2-deficient DCs, such as those from Crohn's disease patients, are defective to engage the IL-23–IL-1–IL-17 axis82. Moreover, mice deficient in NOD1, NOD2 or RIP2 are resistant to EAE, associated with diminished activation of CNS-infiltrating DCs and IL-17 expression from CNS T cells83. Aside from the NOD proteins, NLRP3-mediated inflammasome complex, which activates caspase-1 to process pro-IL-1β and pro-IL-18, has been shown to regulate TH17 cell generation. Deficiency of NLRP3, the downstream adaptor ASC, or caspase-1 ameliorates EAE pathogenesis. Mechanistically, this is linked to the requirement of NLRP3 to promote IL-1 and IL-18 production from DCs for the potentiation of TH17 responses84–86. The role of NLRP3 to mediate DC-dependent TH17 cell responses has also been observed in infection models of Bordetella pertussis and C. albicans87,88. Conversely, hyperactivation of NLRP3 causes excessive IL-1β production from APCs, leading to augmented TH17 cell differentiation and TH17 cell-dominant immunopathology89. Therefore, activation of NLPR3-mediated inflammasome by DCs promotes TH17 cell differentiation via IL-1β and/or IL-18 in autoimmune, infectious and inflammatory models.
Cytokine receptors
In addition to recognizing microbial products through PRRs, DCs sense signals transduced from cytokines. The proinflammatory cytokine TNF-α promotes DC production of IL-23, whereas blocking TNF-α activity suppresses TH17 responses and inflammation in psoriasis patients90. The receptor for stem cell factor (SCF), c-Kit (a receptor tyrosine kinase), is upregulated on DCs by TH17 and TH2-skewing stimuli such as allergens and allergy-inducing adjuvants, but not by TH1-inducing adjuvants91. Upregulation of c-Kit on DCs results in PI3K activation and elevated IL-6 secretion that in turn promotes TH17 and TH2 cell differentiation. DCs expressing nonfunctional c-Kit are unable to induce robust TH17 and TH2 responses and airway inflammation, highlighting the importance of the c-Kit–PI3K–IL-6 signaling axis in DCs in instructing TH17 responses91.
Conversely, IFN-β, an effective therapy against relapsing-remitting multiple sclerosis, has recently been shown to suppress TH17 cell differentiation by altering the production of TH17-polarizing cytokines from DCs92–94. Engagement of type I IFN receptor (IFNAR) by IFN-β on DCs prevents the expression of an intracellular isoform of osteopontin, termed iOpn, an inhibitor of IL-27. Mice containing DC deficient in iOpn produce excessive amounts of IL-27 and develop a delayed EAE disease associated with diminished IL-17 responses. These studies identify an IFNAR-iOpn axis that restrains TH17 cell development93. However, it should be noted that IFN-β is ineffective and might worsen clinical status in multiple sclerosis and other diseases when a TH17 immune response is prominent95, 96. This probably reflects the effects of IFN-β on cells other than DCs, and highlights the complex roles of IFNAR in the pathogenesis and treatment of autoimmune diseases. Interestingly, ligation of IFN-γ receptor (IFNGR) expressed by DCs leads to suppression of IL-17 production while inducing IL-10 from T cells, and this is mediated by the ability of IFN-γ to induce the expression of IL-27 but inhibit that of the secreted form of osteopontin97, 98. Cytokine receptor signaling in DCs therefore shapes differentiation of TH17 cells.
Receptors for additional immune modulators
DCs employ additional receptors to link inflammatory and environmental cues to proper T cell lineage decision. Ligation of CD40 on DCs by T cell-expressed CD40L endows DCs with the ability to promote TH17 cell differentiation via IL-627, 28. Stimulation of the chemokine receptor CCR7 by its ligands promotes the expression of IL-23 from DCs and IL-23-dependent generation of pathogenic TH17 cells in EAE99. Adenosine is an endogenous metabolite produced during hypoxia or inflammation. Recent studies indicate that adenosine acts via A2B adenosine receptor on DCs to promote IL-6 expression and development of TH17 cells100. PGE2, a major lipid mediator released in inflammatory conditions, induces IL-17 production in activated T cells in the model of inflammatory bowel disease (IBD). PGE2 signals through the EP2/EP4 receptors on DCs to shift the IL-12/IL-23 balance in DCs in favor of IL-23101. Analysis of mice deficient in EP2 or EP4 reveals that PGE2 facilitates TH17 cell generation in EAE redundantly through these two receptors102.
As described above, AHR acts in a T cell-intrinsic manner to modulate TH17 cell differentiation20, 21. Interestingly, AHR was recently shown to negatively regulate DC immunogenicity by inducing expression of IL-10 and indoleamine 2,3-dioxygenase (IDO), an immunosuppressive enzyme103. Absence of AHR in DCs inhibits iTreg development and facilitates TH17 cell generation from naïve T cells103, whereas DCs activated with AHR suppresses EAE upon transfer104. Another DC pathway negatively regulating TH17 cell responses is mediated by the Wnt-Frizzled (fzd) receptor and the downstream β-catenin signaling. Manicassamy et al. reported that DC-specific deletion of β-catenin disrupts intestinal homeostasis and results in lower frequency of Treg cells but increased percentages of effector TH17 and TH1 cells105. Deficiency of β-catenin in DCs alters the balance of proinflammatory and anti-inflammatory cytokines and exacerbates the inflammatory response in an IBD model, highlighting that β-catenin signaling in DCs promotes intestinal homeostasis and tolerance105. Therefore, diverse receptors in DCs translate inflammatory mediators and environmental cues into proper TH17 cell generation.
In summary, DCs sense pathogenic agents, immune stimuli and environmental cues through a plethora of receptors. In each case, the ligand and receptor interaction initiates a specific signaling cascade in DCs culminating in the expression of TH17- polarizing cytokines and other factors, which then positively or negatively modulate TH17 cell differentiation. In general, activation of DC receptors induces simultaneous development of multiple T cell subsets, not just TH17 cells alone. Given the well described cross regulation between T cell subsets3–5, whether TH17 cell differentiation is directly or specifically shaped by the signals transduced from DC receptors requires additional studies in many contexts. Moreover, it remains poorly understood how the kaleidoscopes of innate signals are integrated to shape T cell fate decision. Although different mechanisms are likely to exist, we have recently identified that p38α MAPK signaling serves as an important converging point in DCs to integrate these diverse signals to mediate TH17 responses, which is further discussed below.
BRIDGING INNATE AND TH17 IMMUNITY BY p38/MKP-1 SIGNALING IN DCs
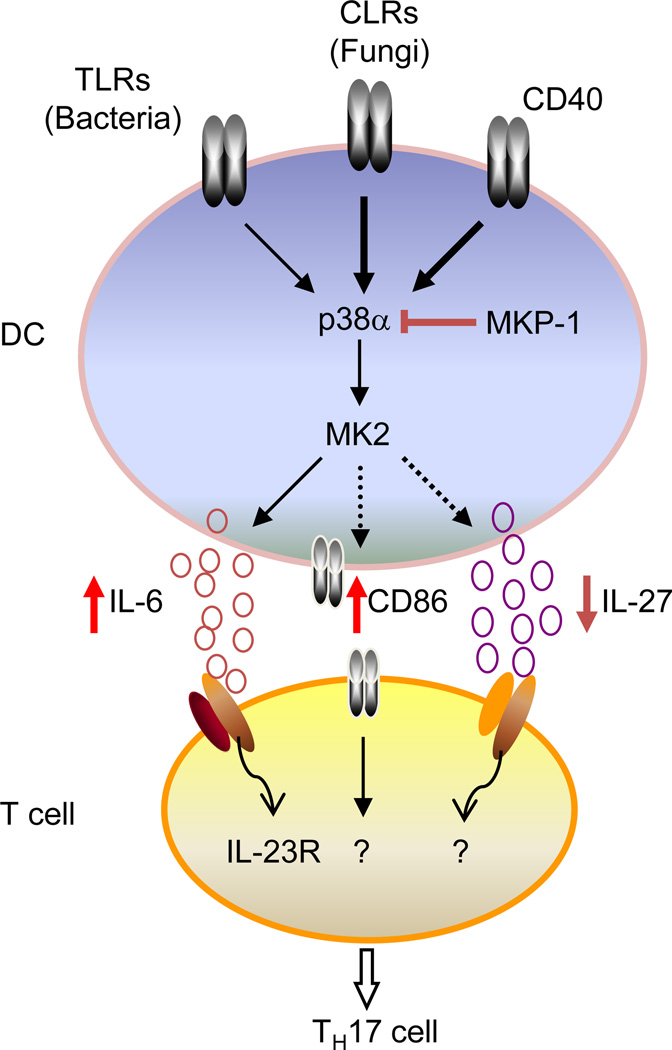
Among the central pathways activated by innate and inflammatory stimuli in DCs is MAPK signaling. MAPKs, comprised of ERK, JNK and p38, represent a fundamental and evolutionarily conserved mechanism for cellular responses to a wide range of extracellular signals106. Excessive activation of MAPKs is associated with many autoimmune and inflammatory diseases, and inhibitors of these pathways have been evaluated as new therapeutics for these disorders107. As a matter of fact, among all of the protein kinase targets for the development of anti-inflammatory drugs in the pharmaceutical industry, p38α MAPK is by far the most extensively investigated protein, but severe side effects have prevented clinical advancement of many p38α inhibitors107. In DCs, p38α is expressed at much higher levels than the other three p38 isoforms (p38β, γ and δ). Importantly, p38 activation is greatly elevated in DCs treated with various types of TH17-instructive signals (including engagements of Dectin-1 and CD40 receptors and stimulation with heat-killed C. albicans), relative to TH1-polarizing stimuli. These findings suggest a potential role for p38α to mediate DC-dependent TH17 cell differentiation by integrating TH17-instructive signals (Figure 3)29.
Figure 3. p38α MAPK/MKP-1 signaling axis programs DCs to regulate TH17 cell differentiation.
p38α in DCs integrates signals from TLRs (e.g., from bacteria), CLRs (e.g., from fungi) and CD40. Following stimulation, p38α activates its downstream targets including MK2 and other factors to regulate the expression of IL-6, IL-27 and CD86, which then deliver the signals to responding T cells for their differentiation into the TH17 lineage. One important target for IL-6 in T cells is IL-23R whose induction further potentiates the TH17 cell differentiation. Conversely, the phosphatase MKP-1 downregulates p38α activity in DCs, thereby suppressing TH17 cell differentiation.
To dissect the selective role of p38α in DCs, we generated mice with DC-specific deletion of p38α and established a central role for p38α to program DC-dependent TH17 cell differentiation and autoimmune CNS inflammation29. p38α in DCs mediates reciprocal regulation of IL-6 and IL-27, arguably the most potent positive and negative regulators of TH17 polarization, respectively, and further imprints STAT3 signaling and IL-23R expression in responding T cells. Additionally, p38α is important for optimal CD86 expression on DCs and shapes strength of the costimulatory signals, thereby orchestrating a program for DC-dependent TH17 cell differentiation. Moreover, p38α is required for tissue-infiltrating DCs to sustain TH17-dependent neuroinflammation, and also contributes to TH17 cell generation in response to commensal microbiota and fungal infection. Mechanistically, p38α integrates diverse TH17-instructive signals (TLRs, CLRs and CD40) in DCs and further links them to a core set of downstream signaling and transcriptional regulators for the expression of DC-derived ‘signals 2 and 3’. These findings identify p38α signaling as a central pathway for the integration of instructive signals in DCs for TH17 cell differentiation and inflammation29.
Negative regulation of MAPK activity is effected primarily by MAPK phosphatases (MKPs), a group of over 10 dual-specificity phosphatases that dephosphorylate the MAPK on their regulatory threonine and tyrosine residues. MKP-1 (DUSP1), the protypical member of this family, is a key negative regulator of innate immune responses by limiting the activation of MAPKs108. DCs lacking MKP-1 exhibit higher activity of p38, and to a lesser extent, JNK71. These mutant DCs show increased ability to drive TH17 cell differentiation but are defective to mediate TH1 cell differentiation, suggesting that MKP-1 signaling in DCs programs reciprocal TH1 and TH17 cell differentiation. This is mediated by the effects of MKP-1 to modulate the IL-12/STAT4 and IL-6/STAT3 axes at the DC–T cell interface, and T cell expression of IL-12Rβ2 and IL-23R (which pair with IL-12Rβ1 to constitute functional IL-12 and IL-23 receptors, respectively). Deficiency of MKP-1 in innate immune cells disrupts in vivo immune responses against infections and immunization and promotes T cell-mediated inflammation. Moreover, MKP-1 inhibits induction of iTreg cells by downregulating TGF-β2 production from DCs. Our findings identify a regulatory circuit linking MKP-1 signaling in DCs, production of immunomodulatory cytokines, and integration of DC-derived signals via STAT activation and cytokine receptor expression in T cells, that bridges innate and adaptive immunity and coordinates protective immunity and immunopathology71.
Notably, whereas MKP-1 and p38α in DCs have reciprocal effects on TH17 cell differentiation, deficiency of MKP-1 also impairs TH1 cell generation but p38α does not play a crucial role in this process29, 71. These results highlight the complex interactions between MKP-1 and p38α. For example, MKP-1 inhibits JNK as well as p38, but also depends upon p38 for its transcriptional induction109, 110. Altogether, our studies identify the MKP-1/p38α axis as a key mechanism of DC-mediated programming of TH17 cell differentiation (Figure 3), and lend strong rationale for therapeutic modulation of this pathway in DCs as a potential treatment for autoimmune conditions mediated by TH17 cells29, 71.
CONCLUDING REMARKS
Dependence of T cell-mediated adaptive immunity on innate immune signals and DCs has been known for a long time111, and the identification of TLRs and other PRRs has revolutionized our understanding of immune responses6. More recent studies have demonstrated that engagements of PRRs and other receptors endow DCs with the ability to shape the differentiation of TH17 cells, one of the most proinflammatory cell types. While recognition of innate and inflammatory stimuli begins at the receptor level, it is the signaling components downstream of each receptor and the way they interact with each other that ultimately determines the specific immunological outcome. The identification of p38α signaling as a key mechanism to integrate TH17-instructive signals in DCs has contributed to our understanding of the crosstalk between innate and adaptive immunity. Many open questions remain in this exciting area. Current studies of TH17 responses are mainly focused on the induction of these cells during inflammatory responses and autoimmune diseases. How DCs are involved in the generation of TH17 cells mediated by commensal microbiota under steady state remains poorly understood66. Similarly, whether DCs regulate the differentiation of TH17 cells in the thymus112, as well as of other IL-17-producing cells such as γδ T cells and innate lymphoid cells113, has yet to be determined. As for the molecular pathways in DCs, the development of CD11c-Cre mice for DC-specific gene targeting has been instrumental to our understanding of DC-mediated innate and adaptive immunity114. We anticipate the extensive use of this approach to address DC pathways for in vivo TH17 responses in the near future. However, given the heterogeneity of DC populations, the CD11c-Cre system does not allow the analysis of all DC subsets in vivo. More sophisticated strategies to target DCs are required to fully appreciate how DC-mediated innate signals mediate adaptive immunity and TH17 cell differentiation. Addressing this issue is not only insightful to understanding fundamental mechanisms of immune regulation, but is also relevant to the investigations of disease mechanisms and therapeutic interventions of a number of autoimmune and inflammatory disorders.
ACKNOWLEDGEMENTS
I acknowledge the large number of researchers who have contributed to this field whose work was not cited owing to space limitations. I thank members of my laboratory for helpful discussions, and Dr. John Lukens for critical reading of the manuscript. The authors’ research is supported by US National Institutes of Health (K01 AR053573 and R01 NS064599), National Multiple Sclerosis Society (RG4180-A-1), Lupus Research Institute, Cancer Research Institute, and the American Lebanese Syrian Associated Charities.
Footnotes
COMPETING INTEREST STATEMENT
The author declares no competing financial interests.
REFERENCES
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 8.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 10.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature reviews. Immunology. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 16.Brustle A, et al. The development of inflammatory T(H)-17 cells requires interferonregulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 17.Schraml BU, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 21.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 22.Shi LZ, et al. HIF1a-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruedl C, Bachmann MF, Kopf M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur J Immunol. 2000;30:2056–2064. doi: 10.1002/1521-4141(200007)30:7<2056::AID-IMMU2056>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Iezzi G, et al. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106:876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perona-Wright G, et al. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–2815. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]
- 29.Huang G, et al. Signaling via the kinase p38alpha programs dendritic cells to drive T(H)17 differentiation and autoimmune inflammation. Nat Immunol. 2012;13:152–161. doi: 10.1038/ni.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odobasic D, Leech MT, Xue JR, Holdsworth SR. Distinct in vivo roles of CD80 and CD86 in the effector T-cell responses inducing antigen-induced arthritis. Immunology. 2008;124:503–513. doi: 10.1111/j.1365-2567.2007.02802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ying H, et al. Cutting edge: CTLA-4--B7 interaction suppresses Th17 cell differentiation. J Immunol. 2010;185:1375–1378. doi: 10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulos CM, et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17- producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 38.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 40.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutcher I, et al. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34:396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veldhoen M, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 49.Melton AC, et al. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 52.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 53.Amadi-Obi A, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 54.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol. 2011;23:438–445. doi: 10.1016/j.smim.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10- producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 57.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 58.Wilke CM, et al. Endogenous interleukin-10 constrains Th17 cells in patients with inflammatory bowel disease. J Transl Med. 2011;9:217. doi: 10.1186/1479-5876-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 60.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 61.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 62.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 65.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnare M, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 68.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdollahi-Roodsaz S, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 71.Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35:45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin Ametabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loures FV, Pina A, Felonato M, Calich VL. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol. 2009;183:1279–1290. doi: 10.4049/jimmunol.0801599. [DOI] [PubMed] [Google Scholar]
- 74.Moreira AP, et al. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. J Clin Invest. 2011;121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lukacs NW, et al. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds JM, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011;32:151–156. doi: 10.1016/j.it.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saijo S, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Fritz JH, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 82.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 83.Shaw PJ, et al. Signaling via the RIP2 adaptor protein in central nervous systeminfiltrating dendritic cells promotes inflammation and autoimmunity. Immunity. 2011;34:75–84. doi: 10.1016/j.immuni.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw PJ, et al. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gris D, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lalor SJ, et al. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 87.Dunne A, et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 88.van de Veerdonk FL, et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011;41:2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaba LC, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishnamoorthy N, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17- mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 95.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Axtell RC, Raman C, Steinman L. Interferon-beta exacerbates Th17-mediated inflammatory disease. Trends Immunol. 2011;32:272–277. doi: 10.1016/j.it.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuwabara T, et al. CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells. J Immunol. 2009;183:2513–2521. doi: 10.4049/jimmunol.0800729. [DOI] [PubMed] [Google Scholar]
- 100.Wilson JM, et al. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J Immunol. 2011;186:6746–6752. doi: 10.4049/jimmunol.1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sheibanie AF, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 102.Esaki Y, et al. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:12233–12238. doi: 10.1073/pnas.0915112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen NT, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quintana FJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manicassamy S, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48:161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 108.Chi H, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim C, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu JH, et al. Feedback control of MKP-1 expression by p38. Cell Signal. 2007;19:393–400. doi: 10.1016/j.cellsig.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Marks BR, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 114.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]