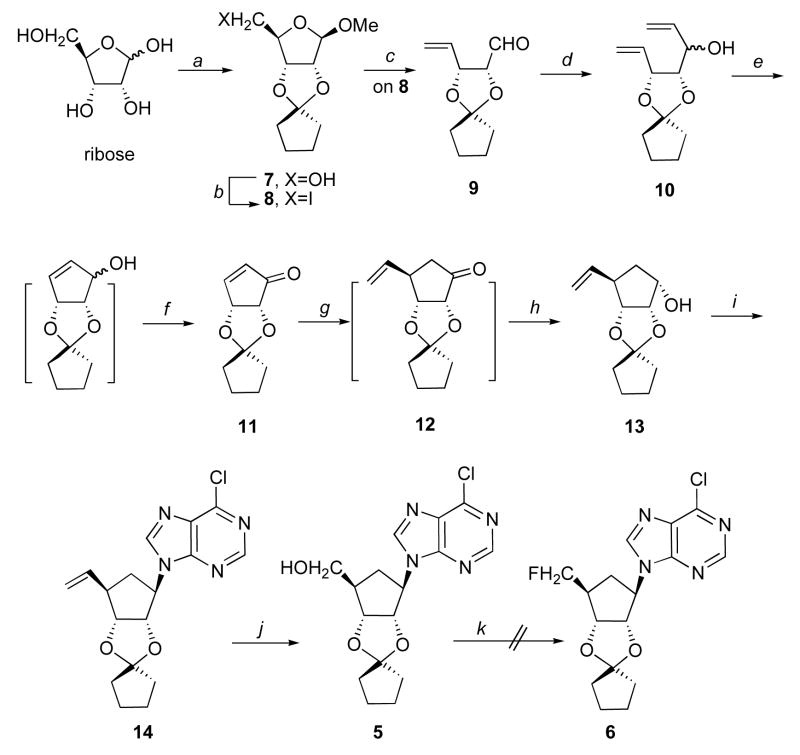
Scheme 1.
Reactions and conditions: a, cyclopentanone, MeOH, (MeO)3CH, 5% pTSA, reflux, 85%; b, I2, imidazole, TPP, tolune/MeCN, reflux, 80%; c, nBuLi, −78 °C, ether, 80%; d, CH2CHMgBr, dry Et2O, −78 °C~rt, 80%; e, 1st gen. Grubbs cat. 1% mol, reflux, CH2Cl2; f, SO3-pyrridine, DIPEA, DMSO, CH2Cl2, 0 °C, 70% two steps; g, CH2CHMgBr, CuBr-Me2S, TMSCl, THF, −78 °C~rt; h, LiAlH4, THF, 62% two steps; i, 6-Cl-purine, DIAD, THF, 60 °C, 2 days, 60%; j, NaIO4, OsO4, MeOH/H2O, then NaBH4, MeOH, 68%; k, one example, DAST, CH2Cl2, reflux.