Abstract
Background
Clinical and epidemiological studies have implicated chronic infections in the development of atherosclerosis. It has been proposed that common mechanisms of signaling via toll like receptors (TLRs) link stimulation by multiple pathogens to atherosclerosis. However, how pathogen specific stimulation of TLR4 contributes to atherosclerosis progression remains poorly understood.
Methods and Results
Atherosclerosis-prone apolipoprotein-E null (ApoE−/−) and TLR4 deficient (ApoE−/−TLR4−/−) mice were orally infected with the periodontal pathogen, Porphyromonas gingivalis. ApoE−/−TLR4−/− mice were markedly more susceptible to atherosclerosis following oral infection with P. gingivalis. Using live animal imaging, we demonstrate that enhanced lesion progression occurs progressively and was increasingly evident with advancing age. Immunohistochemical analysis of lesions from ApoE−/−TLR4−/− mice revealed an increased inflammatory cell infiltrate composed primarily of macrophages and IL-17 effector T cells (Th17), a subset linked with chronic inflammation. Furthermore, enhanced atherosclerosis in TLR4-deficient mice was associated with impaired development of T helper type-1 (Th1) immunity and regulatory T cell (Treg) infiltration. In vitro studies suggest that the mechanism of TLR4-mediated protective immunity may be orchestrated by dendritic cell interleukin (IL)-12 and IL-10, prototypic Th1 and Treg polarizing cytokines.
Conclusions
We demonstrate an atheroprotective role for TLR4 in response to infection with the oral pathogen, P. gingivalis. Our results point to a role for pathogen-specific TLR signaling in chronic inflammation and atherosclerosis.
Keywords: Inflammation, Transgenic/Knockout Mice, Bacterial Infection, Monocytes/Macrophages
Introduction
The identification of atherosclerosis as a chronic inflammatory disease has emphasized the fundamental role of the immune system in disease pathogenesis (1). Detection of endogenous and microbial ligands by immune competent cells occurs via germ-line encoded pattern recognition receptors including the innate immune TLRs (2). Engagement via TLRs initiates acute inflammatory responses that are critical in host defense (3). Resident cells in human atherosclerotic plaque express TLRs (4). Animal studies employing hyperlipidemic mice have shown that TLR2, TLR4, and the downstream signaling molecule, MyD88, play an important role in diet-induced atherosclerosis (5-9). Reduced atherosclerotic development has been observed in TLR4-deficient ApoE−/− mice on high fat Western diet (5, 6, 8). We have previously shown that TLR2 also plays an important role in atherosclerotic progression independent of dietary lipids in hyperlipidemic ApoE−/− mice (7).
A subset of Gram-negative mucosal pathogens including Chlamydia pneumoniae and Porphyromonas gingivalis induce chronic and systemic inflammatory responses associated with atherosclerosis through TLR signaling (10-12). P. gingivalis induces a local inflammatory response that results in oral bone destruction manifested as periodontal disease, an inflammatory disease that affects 100 million people in the U.S (13). In addition to chronic inflammation at the initial site of infection, mounting evidence supports a role for P. gingivalis-mediated periodontal disease as a risk factor for systemic diseases, including atherosclerotic cardiovascular disease as well as diabetes and pre-term birth (10, 11, 14-18). P. gingivalis has been detected in human atherosclerotic plaque (19, 20) and animal models of P. gingivalis infection have validated human studies (21-26).
P. gingivalis induces pro inflammatory responses primarily through fimbriae-mediated signaling via TLR2 that is MyD88-dependent (27). In support of this, we previously demonstrated that a P. gingivalis fimbriae mutant, fimA, failed to accelerate atherosclerosis in ApoE−/− mice (22). Furthermore, we established that TLR2 signaling contributes in part to P. gingivalis-induced atherosclerosis in mice on a normal chow diet (28). In addition to signaling for proinflammatory responses via TLR2, P. gingivalis has developed mechanisms to evade detection and eradication by the immune system (29, 30). One such mechanism is modification of its lipid A, the biological core of bacterial LPS, universally recognized by the TLR4-MD2 complex (31). In response to environmental stimuli (32) and availability of the essential nutrient heme (33), P. gingivalis utilizes enzymes to modify the acylation and phosphorylation of its lipid A, resulting in differential recognition by the TLR4 complex (29, 30). In the present study, we demonstrate the unique ability of P. gingivalis to evade TLR4 signaling while inducing TLR2 dependent pro-inflammatory responses, reveals a protective role for TLR4 in chronic inflammatory atherosclerosis.
Materials and Methods
Mice
Male ApoE−/− and C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). TLR4−/− mice on C57BL/6 background were provided by S. Akira (Osaka University). ApoE−/−TLR4−/− mice were generated in our laboratory. Mouse genotypes were confirmed by PCR and experimental mice were age-matched. Mice were maintained under specific pathogen-free conditions and cared for in accordance with Boston University Institutional Animal Care and Use Committee.
Bacteria
P. gingivalis strain 381 was grown anaerobically on blood agar plates (BBL; Becton Dickinson Co.) and used to seed-innoculate brain heart infusion broth (pH 7.4; BBL) supplemented with yeast extract (BBL), hemin (10μg/ml; Sigma), and menadione (1μg/ml). Colony forming units were standardized at an optical density at 660 nm of 1 (equivalent to 1 × 109 CFU/ml) by spectrometry (ThermoSpectronic Genesys 20). LPS from P. gingivalis 381 was isolated using a modified Tri-Reagent protocol (29).
Oral infection
Three independent experiments were performed with ApoE−/− (Total n=40) and ApoE−/−TLR4−/− (Total n=30) mice and data were pooled. Mice were fed a normal chow diet (Harlan Teklad, Madison WI; Global 2018). Six-week-old male mice were given antibiotics (Sulfatrim) ad libidum in the drinking water for ten days, followed by a two-day antibiotic-free period. 100 μl of P. gingivalis 381 (1×109CFU) suspended in vehicle (2% carboxymethylcellulose in PBS) was topically applied to the buccal surface of the maxillary gingiva five times a week for three weeks (34). Control mice received 100 ul of vehicle. Topical application of P. gingivalis to the buccal surface of the maxillary gingiva five times a week for three weeks induces alveolar bone loss in ApoE−/−mice (28). Mice were euthanized 13 wks after the final oral challenge (24wks of age). This time point is consistent with the time frame used in our prior studies (7, 28, 35).
Magnetic Resonance Angiography (MRA)
MRA of the innominate artery was performed with vertical-bore Bruker 11.7 T Avance spectrometer (Bruker; Billerica, MA) as described (35). Mice were anesthetized with 0.5-2% inhaled isoflurane, placed into a 30mm vertical probe (Micro 2.5) maintained at 23°C. Respiration was monitored using a monitoring and gating system (SA Instruments, Wahkesha, WI). The un-gated 3D gradient echo MRA was acquired with the parameters: Slab thickness = 1.5 cm, flip angle = 45°, repetition time (TR) = 20 ms, echo time (TE) = 2.2 ms, field of view (FOV) = 1.5 × 1.5x 1.5 cm, matrix = 128 × 128 × 128, in-plane, number of average (NEX) = 4. Total scan time was ~ 25 min. Image reconstruction and analysis were performed using Paravision™. The 3D reconstruction of the MRA images was achieved by maximum intensity projection (MIP). The cross sections were chosen at 0.3-0.5mm distance below the subclavian bifurcation. Lumen area was manually defined and calculated with Image J (NIH) by 2 independent observers. Measurement reproducibility had an interclass correlation coefficient of 0.92.
Atherosclerotic plaque assessment
Aortas were harvested and stained with Sudan IV as described (22). Digital micrographs were taken, and total area of atherosclerotic plaque was determined using IPLabs (Scanalytics, Inc) by a blinded observer.
Immunohistochemistry
Mice were euthanized (n=4/group), perfused with 4% paraformaldehyde, and aortic arch with heart tissue was embedded in OCT freezing compound. Five μm serial cryosections were collected every 50 μm in the innominate artery and aortic sinus. In the innominate artery, cryosections were obtained from the region corresponding to the greatest plaque size as revealed by MRA, approximately 0.3 mm below the bifurcation of the innominate and subclavian arteries as described (35). Immunohistochemistry was performed using rat anti-mouse F4/80 (Serotec #MCA497R, Oxford, UK), rat anti-mouse CD4 (BD Biosciences # 550278 and Caltag Laboratories), rat anti-mouse CD8 (BD Biosciences #550281), rat anti mouse TLR2 (eBioscience # 13-9021-80) anti-mouse FOXP3 (Enzo Clone MF333F), rat anti-mouse IL-17 (R&D Systems #MAB2276), or isotype controls (Serotec #MCA1125, Oxford, UK). Biotinylated anti-rat (mouse absorbed) IgG was used as secondary antibody (Vector Laboratories, Inc, Burlingame, CA). Digital micrographs were captured. Nuclei were counterstained with hematoxylin and positive cells were enumerated by microscopy. F4/80+, CD8+ T cells, Foxp3+ T cells, For IL-17+ each from three sections of four the quantitation T cells wereofcounted positive from were staining area, images acquired mice/group. using an Olympus BX41 microscope at 100x magnification and automated color thresholding was performed using ImageJ (NIH).
Flow cytometry
Anti-mouse antibodies included CD3 (BD Biosciences #553062), CD4 (BD Biosciences #553049, CD8 (BD Biosciences #553034), Ly6G, CD45 (BD Biosciences #550994) and isotype controls (BD Pharmingen) and IFN-γ (eBioscience #48-7311), IL-17A (eBioscience #51-7177), F4/80 (eBioscience #12-4801), and TLR2 (eBioscience #12-9022). Intracellular cytokine staining was performed using a mouse kit (BD Pharmingen # 559311). Samples were acquired on a BD LSR II flow cytometer (Becton Dickinson) and data were analyzed using FlowJo software (TreeStar, Inc.).
Cell culture
For isolation of bone marrow derived dendritic cells (DCs), bone marrow cells were cultured in RPMI 1640 containing 10% FBS, 1x non-essential amino acids (MP Biomedicals), 50μM beta-mercaptoethanol (Gibco), 100 μg/ml streptomycin/100 IU penicillin (Cellgro), and 20 ng/ml recombinant mouse GM-CSF (Peprotech, Inc.) for 11 days. DCs were greater than 95% positive for CD11c. Non-adherent DCs were collected and re-plated in 24-well dishes at 2×105/cells/well in complete media without antibiotics before addition of P. gingvalis at MOI 25 and 50. After 24 hours, culture supernatants were collected, samples were clarified by centrifugation, and stored at −80 °C for ELISA.
ELISA
Concentrations of IL-6, IL-12p40, and IL-10 in cell culture supernatants were determined by ELISA (BD OptEIA). Plasma was collected from a subset of experimental mice 16 wks post-infection and assayed by ELISA for P. gingivalis-specific antibody isotypes IgG1, IgG2b, IgG2c, and IgG3 as described (36) as follows. Bacteria were washed three times in PBS and fixed overnight at 4°C in 4% paraformaldehyde. Fixed-bacteria were washed 5x in PBS and protein concentration estimated by bicinchoninic acid (BCA) protein assay. Immulon 4HXB plates were coated overnight at 4°C with 10 μg/ml P. gingivalis suspension in PBS containing 0.05% sodium azide. Serial dilutions of mouse serum were plated and determination of IgG isotypes was conducted using the C57BL/6 Clonotyping Kit (SouthernBiotech). Quantitation of IgG was determined using a standard curve. ELISAs were developed using 4-Methylumbelliferyl phosphate (MUP) and read on spectrofluorometer (BioTek Synergy HT).
Splenocyte re-stimulation assay
Splenocytes (2×106/ml) were collected and stimulated with P. gingivalis soluble antigens (10μg/ml) in the presence of 1μg/ml anti-mouse CD28 (eBioscience) for 4 hrs and 10 μg/ml Brefeldin A (eBioscience). Cells were harvested, stained with anti-mouse antibodies (BD Pharmingen): CD3, CD4, and CD8. Intracellular cytokine staining for IL-17A (eBioscience) and IFN-γ (eBioscience) was performed using BD Cytofix/Cytoperm kit.
Statistics
Normality of data was determined by visually inspecting for bell-shaped curves. A Mann-Whitney U test was performed to compare two independent samples with Prism 5 software (GraphPad Software Inc., San Diego, CA) with an alpha equal to 0.05 as considered significant. Two-way ANOVA was performed for analysis of % plaque between genotypes and infections. A value of p< 0.05 was considered significant.
Results
TLR4 deficiency confers enhanced susceptibility to chronic and progressive atherosclerosis following infection with P. gingivalis
ApoE−/− and ApoE−/−TLR4−/− mice were orally infected with 109 CFU P.gingivalis strain 381. The predominant lipid A species expressed by P. gingivalis 381 grown under standard laboratory conditions in the presence of excess heme was tetra-acylated nonphosphorylated (m/z 1380), which is TLR4 inert and immunologically silent (data not shown) (32). The minor lipid A species produced by strain 381 was penta-acylated monophosphorylated (m/z 1690) and has been demonstrated to act as both a weak TLR4 agonist and antagonist (29).
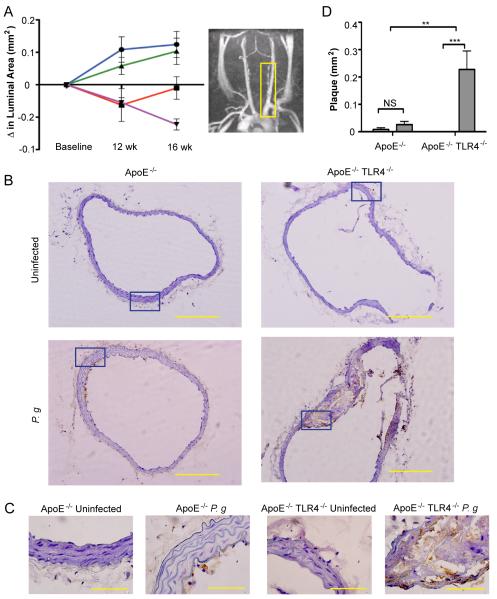
Progression of atherosclerosis in the innominate artery of individual mice was examined in vivo by magnetic resonance angiography (MRA). The innominate artery exhibits a high degree of lesion progression and expresses features of human disease including vessel narrowing, perivascular inflammation, and plaque disruption (35). The luminal area of the innominate artery of P. gingivalis-infected ApoE−/− and ApoE−/−TLR4−/− mice decreased between baseline and 12 weeks compared to uninfected ApoE−/− and ApoE−/−TLR4−/− mice (Fig. 1A) illustrating vessel narrowing and disease progression in infected mice. In P. gingivalis infected ApoE−/− mice, luminal area remained unchanged between 12 and 16 weeks. However, P. gingivalis infected ApoE−/−TLR4−/− mice exhibited a progressive decline in luminal area at 0, 12, and 16 weeks following the first oral infection, indicative of progressive atherosclerosis. Luminal area was significantly smaller in ApoE−/−TLR4−/− mice compared to ApoE−/− mice at 16 weeks (p=0.03).
Figure 1. TLR4 deficiency confers enhanced susceptibility to atherosclerosis in the innominate artery following infection with P. gingivalis.
Innominate arteries were imaged by MRA at baseline (week 0), 12, and 16 wks following first oral infection. (A) The temporal change in luminal area (mm2) was calculated for individual mice (n=5/group). Inset, representative MRA image indicating the innominate artery (yellow box), where measurements were taken. Uninfected ApoE−/− (blue); P. gingivalis infected ApoE−/− (red); Uninfected ApoE−/− TLR4−/− (green); P. gingivalis infected ApoE−/−TLR4−/− (purple). (B) Representative hematoxylin staining from each group in innominate artery with F4/80 staining (macrophages stain brown). Scale=20μm. (C) Visualization of intima, media and adventitia of representative images. Area indicated in 1C (blue box). Scale=5μm. (D) Plaque area within the innominate artery measured from histological images using IPLab software (Becton, Dickinson and Company). (n=5/group). **p<0.01, ***p<0.001. White bar: Uninfected. Gray bar: P. gingivalis infected.
Assessed by microscopy in post-mortem sections, the innominate artery of uninfected ApoE−/− and ApoE−/−TLR4−/− mice appeared as tightly packed layers of smooth muscle cells with uniform distribution about the circumference of the artery with no apparent lipids and inflammatory cells (Fig. 1B: ApoE−/−, upper left; ApoE−/−TLR4−/−, upper right). Brown staining indicates the presence of macrophages. Plaque formation in the innominate artery of P. gingivalis infected ApoE−/− mice was modest and superficial, appearing as fatty streaks at the intimal surface (Fig. 1B ; lower left). In P. gingivalis infected ApoE−/−TLR4−/− mice, we observed a significant increase in arterial plaque, which accumulated within subendothelial layers and coincided with the infiltration of inflammatory cells, including macrophages (Fig. 1B; lower right). In contrast to fatty streaks in infected ApoE−/− mice, plaques in infected ApoE−/−TLR4−/− mice protruded into the arterial lumen. Higher resolution reveals the structure of the intima, media and adventitia in the innominate arteries of each group (Fig. 1C). Corresponding sections from MRA analyses revealed an increase in plaque area within the innominate artery of infected ApoE−/−TLR4−/− mice compared to infected ApoE−/− mice (Fig. 1D bar graph). No significant differences in plaque area between uninfected ApoE−/− and ApoE−/−TLR4−/− mice were observed.
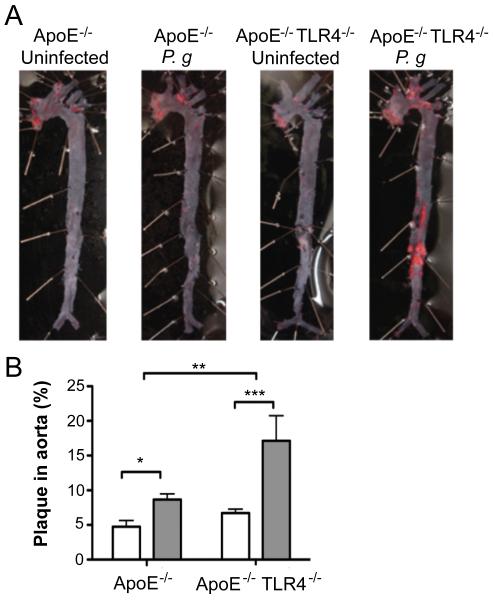
In the absence of infection, no differences in en face total aortic lesion area, assessed by lipid staining, were observed between uninfected ApoE−/− and ApoE−/−TLR4−/− mice (Fig. 2A and 2B). Consistent with our previous studies (22), infected ApoE−/− mice developed significantly more plaque than uninfected ApoE−/− controls. Aortas from infected ApoE−/−TLR4−/− mice also demonstrated significantly more plaque than their uninfected, genotype-matched ApoE−/−TLR4−/− controls; however, plaque area was significantly greater in infected ApoE−/−TLR4−/− mice compared to infected ApoE−/− mice. While the increase in lesion area in infected ApoE−/− mice largely localized to the atherosclerosis prone regions in the aortic arch, lesions in infected ApoE−/−TLR4−/− mice occurred in the proximal as well as the distal aorta.
Figure 2. TLR4 deficiency confers enhanced susceptibility to atherosclerosis in the aorta following infection with P. gingivalis.
(A) Sudan IV staining of aorta en face lesions 16 wks following first infection with P. gingivalis. (B) Quantification of lipid content within the total aorta of uninfected (white bars) and P. gingivalis infected mice (gray bars) (n=10-13/group). Percentage of aorta occupied by lipids was calculated using IPLab software (Becton, Dickinson and Company). *p<0.05, ***p<0.001. **p< 0.01.
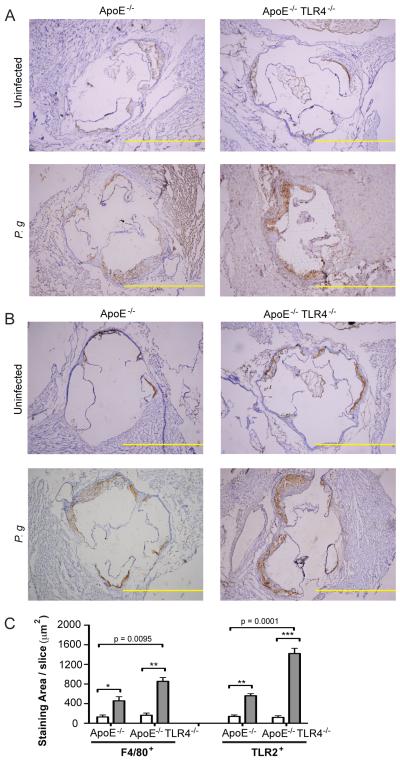
TLR4 deficiency is associated with increased macrophage infiltration and expression of TLR2 in aortic lesions from P. gingivalis infected mice
The increased atherosclerotic plaque observed in infected ApoE−/−TLR4−/− mice was accompanied by a significantly increased accumulation of macrophages within the aortic sinus while macrophage accumulation was not significantly increased in infected ApoE−/− mice (Fig. 3A and 3C, left). In agreement with previous findings (22), P. gingivalis infection resulted in increased expression of TLR2 within the aortic sinus of infected ApoE−/− mice, as well as in ApoE−/−TLR4−/− mice, in areas where macrophages were found (Fig. 3B and 3C, right). TLR2 expression was also significantly higher in infected ApoE−/−TLR4−/− mice compared to ApoE−/− mice (Fig. 3C, right).
Figure 3. TLR4 deficiency is associated with increased macrophage influx and TLR2 expression in atherosclerotic plaques following infection with P. gingivalis.
Aortic sinus sections from uninfected and P. gingivalis infected ApoE−/− and ApoE−/−TLR4−/− mice were stained for F4/80 and TLR2 positive cells with hematoxylin counterstaining. Representative aortic sinus sections stained for (A) F4/80 and (B) TLR2. Scale=100μm. (C) Quantification of positive staining area using ImageJ software (NIH). White bar: Uninfected. Gray bar: P. gingivalis infected. Three sections from n=4/group were analyzed. Data are mean ± SD positive staining area/slice. Intra-genotype comparisons were calculated by Mann Whitney U test. * p<0.05, **p<0.01, ***p<0.001. Inter-genotype comparisons were calculated by Two-way ANOVA, p=0.0001, p=0.0095.
Greater plaque area and infiltration of macrophages into plaque in ApoE−/−TLR4−/− mice cannot be attributed to differences in plasma cholesterol or triglycerides, as these were similar among all groups (Cholesterol, mean+SE: uninfected ApoE−/−, 476+22; infected ApoE−/−, 449+24; uninfected ApoE−/−TLR4−/−, 500+36; infected ApoE−/−TLR4−/−, 512+24 mg/dL. Triglycerides, mean+SE: uninfected ApoE−/−, 237+21; infected ApoE−/−, 245+18; uninfected ApoE−/−TLR4−/−, 225+24; infected ApoE−/−TLR4−/−, 206+22 mg/dL).
TLR4 deficiency promotes Th17/Treg imbalance in atherosclerotic lesions following infection with P. gingivalis
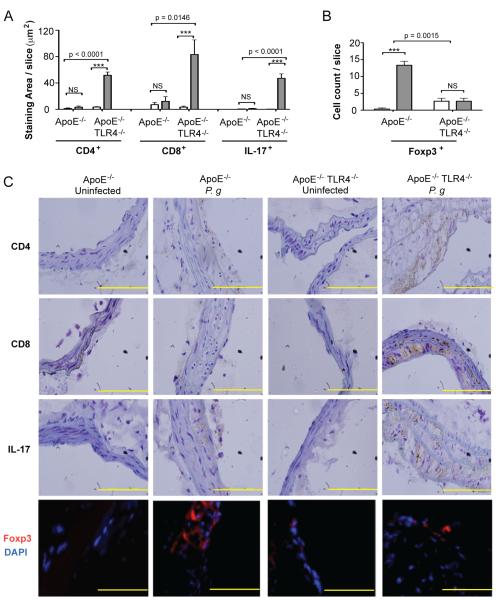
In infected ApoE−/− mice, we observed no increase in CD8+ T cells, TCD4+ T cells or IL-17+ cells in the innominate artery, compared to uninfected ApoE−/− mice (Fig. 4A and 4C). Accumulation of CD4+ and CD8+ cells within the innominate artery of infected ApoE−/−TLR4−/− mice was dramatically increased compared to infected ApoE−/− mice. The abundance of T cells was accompanied by increased numbers of IL-17 expressing cells and markedly diminished numbers of Foxp3+ expressing regulatory T cells (Treg cells) (Fig. and 4C). The marked increase in CD4+, CD8+ and IL-17+ cells and the diminution of FoxP3+ 4B Treg cells in infected mice in the absence of TLR4 expression (ApoE−/−TLR4−/− mice) reveal that in the presence of TLR4 expression, TLR4 may be protective following P. gingivalis infection that serves to prevent the infiltration of IL-17+ T cells and enhance the numbers of Foxp3+ Treg cells in the inflammatory lesion.
Figure 4. TLR4 deficiency promotes Th17/Treg imbalance in atherosclerotic lesions following infection with P. gingivalis.
Quantitative immunohistochemistry of (A) CD4, CD8, IL-17 positive staining area in the innominate artery of uninfected and P. gingivalis infected ApoE−/− and ApoE−/−TLR4−/− mice using ImageJ software (NIH) or (B) Foxp3 positive cell count. Three sections from n=4 mice/group were analyzed. White bar: Uninfected. Gray bar: P. gingivalis infected. Data are mean ± SD of positive staining area/slice or cell count/slice. Intra-genotype comparisons were calculated by Mann Whitney U test ***p<0.0001. NS= not significant. Inter-genotype comparisons were calculated by Two-way ANOVA (indicated p values). (C) Representative immunohistochemistry of CD4, CD8, IL-17, and Foxp3 positive cells in the innominate artery of uninfected and P. gingivalis infected ApoE−/− and ApoE−/−TLR4−/− mice. Scale=5μm.
IgG humoral immunity and Th1 responses are altered in the absence of TLR4
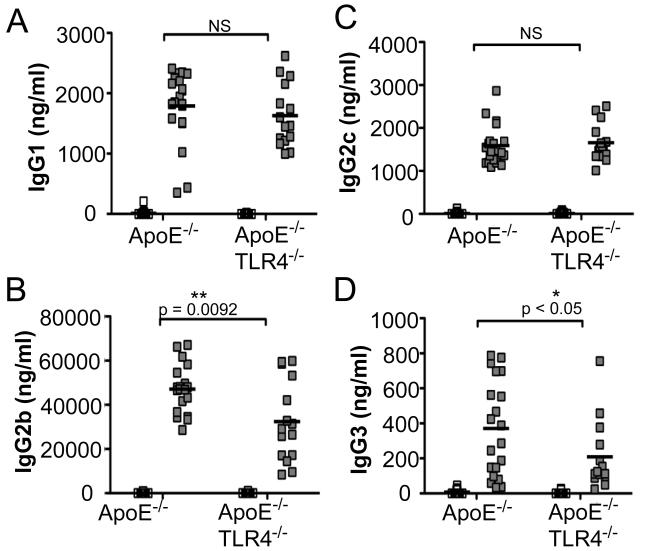
Infection with P. gingivalis induced a robust IgG1 response in both ApoE−/− and ApoE−/−TLR4−/− mice, indicating preservation of IgG1-mediated humoral immunity in the absence of TLR4 (Fig. 5A). However, P. gingivalis-infected ApoE−/−TLR4−/− mice produced significantly reduced IgG2b (Fig. 5B) and IgG3 (Fig. 5D) responses compared to ApoE−/− mice, IgG subclasses that are associated with Th1 responses (37). IgG2c levels were increased to a similar level in infected ApoE−/− and ApoE−/−TLR4−/− mice (Fig. 5C).
Figure 5. P. gingivalis-specific antibody isotypes IgG1, IgG2b, IgG2c, and IgG3.
P. gingivalis-specific IgG production in uninfected ApoE−/− (n=15), P. gingivalis infected ApoE−/− (n=21), uninfected ApoE−/−TLR4−/− (n=17) and P. gingivalis infected ApoE−/−TLR4−/− (n=14) mice as measured by ELISA. (A) IgG1, (B) IgG2b, (C) IgG2C, (D) IgG3. White squares= uninfected, Gray squares= P. gingivalis infected. Data were analyzed by Student’s t-test. *p<0.05, **p=0.0092. NS=not significant.
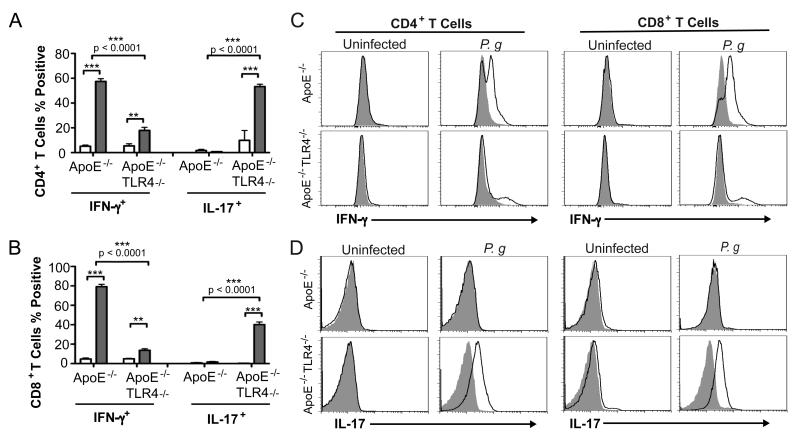
We restimulated splenocytes from experimental mice with P. gingivalis soluble antigens and identified responsive cells that express the effector cytokines IFN-γ and IL-17. T cells from uninfected mice did not exhibit cytokine expression in response to P. gingivalis antigens. We observed a high percentage of IFN-γ expressing CD4+ (Fig. 6A and 6C) and CD8+ (Fig. 6B and 6C) T cells from P. gingivalis infected ApoE−/− mice. In contrast, the majority of responsive CD4+ (Fig. 6A and 6D) and CD8+ T cells (Fig. 6B and 6D) from infected ApoE−/−TLR4−/− mice expressed IL-17. A small subset of CD8+ (14%) T cells from infected ApoE−/−TLR4−/− mice also expressed IFN-γ. Although the number of reactive T cells indicates that these responses may not be antigen specific, they were specific to P. gingivalis infection, as T cells from uninfected mice failed to respond to stimulation with antigens. These results suggest that in the absence of TLR4, P. gingivalis infection results in impaired Th1 immunity and IL-17 skewing.
Figure 6. Impaired Th1 immunity and Th17 skewing in P. gingivalis infected TLR4-deficient mice.
Expression of IFN-γ and IL-17 in (A) CD4+ and (B) CD8+ splenic T cells from uninfected and P. gingivalis infected mice following in vitro stimulation with 10 μg/ml P. gingivalis soluble antigens and 1 μg/ml anti-mouse CD28 for 4h in the continuous presence of Brefeldin A. Data represent mean ± SD, n=4/group. White bar= uninfected, Gray bar= P. gingivalis infected. Intra-genotype comparisons were calculated by Mann Whitney U test. **p<0.01, ***p<0.001. Inter-genotype comparisons were calculated by Two-way ANOVA with Bonferonni’s post-test. (C) Representative flow cytometry gating for the analysis of cytokine secreting CD4+ and CD8+ T cells.
Th1 and Treg polarizing cytokine production following P. gingivalis infection is impaired in dendritic cells from TLR4 deficient mice
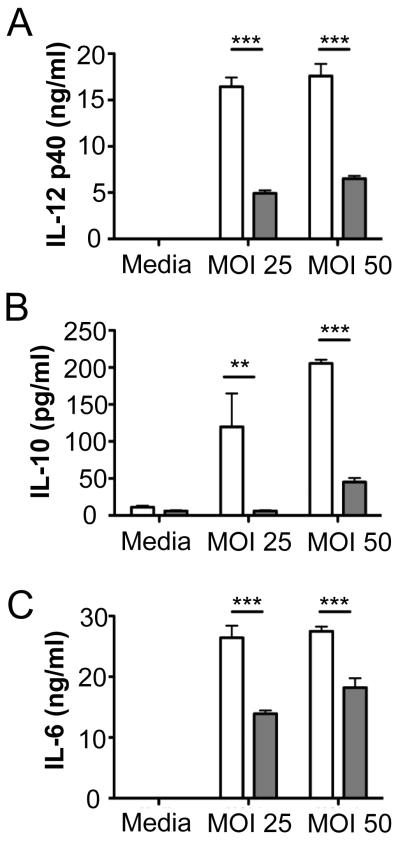
Activation of TLRs on dendritic cells (DC) triggers the release of cytokines that play decisive roles in modulating T helper subset differentiation from naïve CD4+ cells (38). To investigate the role of P. gingivalis-induced TLR4 activation in DC production of T cell polarizing cytokines, DCs from wild type and TLR4−/− mice were infected with P. gingivalis and expression of T cell polarizing cytokines was examined. P. gingivalis induced the production of IL-12 (Fig. 7A), IL-10 (Fig. 7B), and IL-6 (Fig. 7C), in DCs from ApoE−/− mice. Production of these cytokines was markedly reduced in DCs from ApoE−/− TLR4−/− mice. These results suggest that TLR4 is necessary for production of these cytokines following P. gingivalis infection. The abrogated DC production of T cell polarizing cytokines in the absence of TLR4 may be responsible for impaired development of Th1/Treg effector immunity as well as the enhanced IL-17 expression in T cell populations within plaque of ApoE−/−TLR4−/− mice.
Figure 7. Th1 and Treg polarizing cytokine production following P. gingivalis infection is impaired in dendritic cells from TLR4 deficient mice.
Bone marrow derived dendritic cells from C57BL/6 (white bars) and TLR4−/− (gray bars) mice were infected with P. gingivalis at the indicated multiplicity of infection (MOI). After 24h, supernatants were analyzed for (A) IL-12, (B) IL-10, and (C) IL-6 by ELISA. Bars represent mean ± SD from triplicate cultures. **p<0.01, ***p<0.001 by Student’s t-test.
Discussion
Common chronic infections may contribute to up to 40% of newly developed atherosclerotic cases (39). A role for P. gingivalis-mediated periodontal disease as a risk factor for atherosclerotic cardiovascular disease is well documented (10, 11, 14-18). The observation that innate immune signaling triggered by P. gingivalis is dysregulated within atherosclerotic lesions has sparked interest in the association between oral infection and induction of innate immune cascades in atherosclerosis progression (40). Most experimental studies have focused on the proatherogenic consequence of TLR signaling in mouse models of atherogenesis; many involving the influence of high fat diet (5, 6, 8, 12). In contrast to studies reporting diminished high fat diet-induced atherosclerosis in TLR4 deficient mice, we report the unexpected finding that TLR4-deficient mice are markedly more susceptible to atherosclerosis following infection with P. gingivalis. Live animal imaging demonstrated that enhanced disease severity occurred progressively, long after cessation of the infectious stimulus and at two anatomically relevant sites, in large (aortic sinus) and medium (innominate artery) sized vessels. Enhanced atherosclerosis progression in ApoE−/−TLR4−/− mice compared to ApoE−/− mice is unlikely to be due to differences in plasma cholesterol or triglycerides, which were similar among all groups. Minimal atherosclerotic lesion area in the innominate artery was observed in uninfected ApoE−/− mice and this is likely due to the fact that animals were fed a normal chow diet. In our recent study in which atherosclerosis progression was examined using MRA in the innominate artery of uninfected and P. gingivalis-infected ApoE−/− mice, animals were fed a high fat diet for the duration of the study (35). High fat diet enhances atherosclerosis progression in ApoE−/− mice. In the absence of high fat diet and infection, plaque accumulation within the aorta and innominate artery progresses more slowly and is minimal at the time point examined in the present study. Effective control of immune-mediated pathology in P. gingivalis infected ApoE−/−mice coincided with an increase in Treg within the innominate artery. In contrast, the exacerbated inflammatory pathology in P. gingivalis infected ApoE−/−TLR4−/− mice was associated with increased lesion macrophage numbers, T cell infiltration and enhanced expression of IL-17. Regulatory T cells (Tregs) play a critical role in maintaining immunological tolerance and controlling the extent of immune-mediated pathology, especially in cases of chronic infection (41, 42). Our studies indicate that in the absence of TLR4, mice fail to develop protective Th1 immunity and were unable to regulate adaptive immune responses mediated by Th17 cells following P. gingivalis infection. We propose that this results in a breakdown of immunological tolerance, owing to impaired Treg function, leading to unrestricted activation of pathogenic T cells that mediate arterial inflammation. The unique TLR4 evasive properties of P. gingivalis lipid A position this organism to disrupt effector T cell mechanisms at the level of dendritic cell activation, the interface of innate and adaptive immunity.
TLR2 expression was increased markedly in aortic lesions by P. gingivalis infection in ApoE−/− mice and further increased in ApoE−/−TLR4−/− mice. It is plausible that enhanced TLR2 expression in ApoE−/−TLR4−/− mice may have contributed to increased vascular inflammation and atherosclerosis in ApoE−/−TLR4−/− mice. Thus, the increase in atherosclerosis in ApoE−/− TLR4−/− mice may be a result of not only TLR4 deficiency, but also high TLR2 expression. This increased TLR2 expression in activated macrophages and the endothelium may reflect the development and maintenance of a hyper-inflammatory state in the absence of TLR4 expression. This observation was an unexpected finding of this study, as was the finding that plaque development was enhanced in the absence of TLR4.
Our results also showed that following in vitro restimulation with antigen, T cells from P. gingivalis-infected ApoE−/−TLR4−/− mice predominantly produced IL-17, while IFN-gamma was the predominant cytokine produced by T cells from infected ApoE−/− mice. We also demonstrate that TLR4-deficiency was associated with markedly inhibited production of the Th1 polarizing cytokine, IL-12 by P. gingivalis-infected dendritic cells. Taken together, our findings suggest that an initially impaired Th1 response in TLR4-deficient mice results in Th17 skewing of the adaptive immune response, which may be the mechanism for exacerbated atherosclerosis. Indeed, research supports a complex relationship between the Th1 and Th17 cell lineages, and many T cells expressing IL-17 co-express IFN-gamma. We previously reported that IFN-gamma is significantly up-regulated at the protein level in atherosclerotic lesions from P. gingivalis-infected ApoE−/− mice relative to uninfected controls (28). This finding is in agreement with IFN-gamma contributing to the development of atherosclerosis as suggested by one study (43). Several studies demonstrate that both IFN-gamma and IL-17 may be pro-atherogenic in mouse models, and circulating levels of both of these cytokines are increased in patients with coronary atherosclerosis (44). A large body of literature also supports a pro-atherogenic role for IL-17 in mouse models of atherosclerosis, and neutralization of IL-17 has been demonstrated to reduce pathogen- and diet-induced atherosclerosis in ApoE−/− mice (45). These studies demonstrate that the development of atherosclerosis is multifactorial and may be influenced by the inciting stimulus (i.e., high fat diet vs. pathogen-mediated).
Notably, humans are specifically impaired in their ability to recognize penta-acylated lipid A, a phenomenon reflecting bacterial adaptation to the human host (46). Species-specific discrimination of atypical penta-acylated lipid A is mediated by a hypervariable 82-amino acid sequence in the middle region of TLR4, a region where human polymorphisms are common (31, 46). Our results provide a mechanistic link regarding the conflicting reports on the association of human TLR4 polymorphisms and atherosclerotic diseases. Thus, although we only see a hyper inflammatory phenotype in TLR4-deficient mice, it is plausible that common human TLR4 polymorphisms (47-49) that attenuate receptor signaling may predispose individuals to an increased risk of atherosclerosis associated with bacterial infection, which is in contrast to atherosclerosis risk associated with a Western high fat diet.
Recent studies reported that ApoE−/−TLR4−/− mice fed a high fat diet and infected intranasally with C. pneumoniae exhibited diminished atherosclerosis compared to infected ApoE−/− mice (12). A separate report demonstrated a protective role for TLR4 deficiency in diet-induced atherogenesis (8). It is important to note that these results could not be recapitulated under germ-free conditions (50), indicating a potential interaction between hyperlipidemia and indigenous microbes. Based on these observations it was proposed that common mechanisms of signaling via TLR2, TLR4 and MyD88 link stimulation by multiple pathogens and endogenous ligands to atherosclerosis, and that therapeutic TLR4 antagonism could prove beneficial in the treatment of chronic atherosclerosis (8, 51, 52). Our results clearly point to a critical role for specific TLR signaling, in particular, TLR4, in chronic inflammation and atherosclerosis induced by P. gingivalis. Our results raise caution for the safety and efficacy of TLR4 antagonists for the treatment of atherosclerosis, especially in patients with co-morbid conditions including periodontal disease and other infectious diseases.
Acknowledgements
We thank Dr. Stephen R. Coates for analysis of bacterial lipid A (Department of Periodontics, University of Washington). We thank Dr. Xuemei Zhong and Nathalie Bitar (Boston University Immunohistochemistry Core) for technical assistance.
Footnotes
Disclosures
None
This work was supported by National Institutes of Health grant NIH NHLBI R01 HL080387 (C.A.G.) and NIH NIAID P01 AI078894 (C.A.G).
References
- 1.Cole JE, Georgiou E, Monaco C. The Expression and Functions of Toll-Like Receptors in Atherosclerosis. Mediators of Inflammation. 2010;2010:1–18. doi: 10.1155/2010/393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgkinson C, Ye S. Toll-Like Receptors, Their Ligands, and Atherosclerosis. The Scientific World JOURNAL. 2011;11:437–453. doi: 10.1100/tsw.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 4.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 5.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004 doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 6.Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:50–57. doi: 10.1161/ATVBAHA.110.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. The Journal of Clinical Investigation. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson FC, 3rd, Yumoto H, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi C, Gudino CV, Gibson FC, 3rd, Genco CA. Pathogen-Induced Chronic Inflammation at Sites Distant from Oral Infection: Bacterial Persistence and Modulation of Cell Specific Innate Immune Inflammatory Pathways. Mol Oral Microbiol. 2010;25:305–316. doi: 10.1111/j.2041-1014.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 14.Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol. 2003;23:1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- 15.Dasanayake AP, Russell S, Boyd D, Madianos PN, Forster T, Hill E. Preterm low birth weight and periodontal disease among African Americans. Dent Clin North Am. 2003;47:115–125. x–xi. doi: 10.1016/s0011-8532(02)00056-3. [DOI] [PubMed] [Google Scholar]
- 16.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 17.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 18.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 19.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 20.Padilla C, Lobos O, Hubert E, Gonzalez C, Matus S, Pereira M, Hasbun S, Descouvieres C. Periodontal pathogens in atheromatous plaques isolated from patients with chronic periodontitis. J Periodontal Res. 2006;41:350–353. doi: 10.1111/j.1600-0765.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 21.Amar S, Wu SC, Madan M. Is Porphyromonas gingivalis cell invasion required for atherogenesis? Pharmacotherapeutic implications. J Immunol. 2009;182:1584–1592. doi: 10.4049/jimmunol.182.3.1584. [DOI] [PubMed] [Google Scholar]
- 22.Gibson FC, 3rd, Hong C, Chou H-H, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 23.Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, Lu Y, Papapanou PN, Schmidt AM. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein e-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1405–1411. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Messas E, Batista EL, Jr., Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 25.Madan M, Amar S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS ONE. 2008;3:e3204. doi: 10.1371/journal.pone.0003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto T, Yumoto H, Takahashi Y, Davey M, Gibson FC, 3rd, Genco CA. Pathogen-accelerated atherosclerosis occurs early after exposure and can be prevented via immunization. Infect Immun. 2006;74:1376–1380. doi: 10.1128/IAI.74.2.1376-1380.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajishengallis G, Wang M, Liang S. Induction of Distinct TLR2-Mediated Proinflammatory and Proadhesive Signaling Pathways in Response to Porphyromonas gingivalis Fimbriae. J Immunol. 2009;182:6690–6696. doi: 10.4049/jimmunol.0900524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi C, Madrigal AG, Liu X, Ukai T, Goswami S, Gudino CV, Gibson FC, 3rd, Genco CA. Pathogen Mediated Inflammatory Atherosclerosis is Mediated in Part via TLR2 Induced Inflammatory Responses. J Innate Immun. 2010;2:334–343. doi: 10.1159/000314686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coats SR, Berezow AB, To TT, Jain S, Bainbridge BW, Banani KP, Darveau RP. The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria. Infect Immun. 2011;79:203–210. doi: 10.1128/IAI.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol. 2009;11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nature Reviews Microbiology. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 32.Curtis MA, Percival RS, Devine D, Darveau RP, Coats SR, Rangarajan M, Tarelli E, Marsh PD. Temperature-dependent modulation of Porphyromonas gingivalis lipid A structure and interaction with the innate host defenses. Infect Immun. 2011;79:1187–1193. doi: 10.1128/IAI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun. 2006;74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Archives of Oral Biology. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi C, Viereck J, Hua N, Phinikaridou A, Madrigal AG, Gibson FC, 3rd, Hamilton JA, Genco CA. Porphyromonas gingivalis accelerates inflammatory atherosclerosis in the innominate artery of ApoE deficient mice. Atherosclerosis. 2011;215:52–59. doi: 10.1016/j.atherosclerosis.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson FC, 3rd, Gonzalez DA, Wong J, Genco CA. Porphyromonas gingivalis-Specific Immunoglobulin G Prevents P. gingivalis-Elicited Oral Bone Loss in a Murine Model. Infect Immun. 2004;72:2408–2411. doi: 10.1128/IAI.72.4.2408-2411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, K√a∂lsch E, Podlaski FJ, Gately MK, R√°de E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. European Journal of Immunology. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 38.Peters M, Dudziak K, Stiehm M, Bufe A. T-cell polarization depends on concentration of the danger signal used to activate dendritic cells. Immunology and Cell Biology. 2010;88:537–544. doi: 10.1038/icb.2010.3. [DOI] [PubMed] [Google Scholar]
- 39.Kiechl S, Egger G, Mayr M, Wiedermann CJ, Bonora E, Oberhollenzer F, Muggeo M, Xu Q, Wick G, Poewe W, Willeit J. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103:1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Ait-Oufella H, Salomon BÆL, Potteaux S. p., Robertson A-KL, Gourdy P, Zoll J, Merval R. g., Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson G. r. K., Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nature Medicine. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 42.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science (New York, N.Y.) 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nature Immunology. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 47.Ajdary S, Ghamilouie M-M, Alimohammadian M-H, Riazi-Rad F, Pakzad S-R. Toll-like receptor 4 polymorphisms predispose to cutaneous leishmaniasis. Microbes and Infection/Institut Pasteur. 2011;13:226–231. doi: 10.1016/j.micinf.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 49.Montes AH, Asensi V, Alvarez V, Valle E, Oca√±a MG, Meana A, Carton JA, Paz J, Fierer J, Celada A. The Toll-like receptor 4 (Asp299Gly) polymorphism is a risk factor for Gram-negative and haematogenous osteomyelitis. Clinical and Experimental Immunology. 2006;143:404–413. doi: 10.1111/j.1365-2249.2005.03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright SD, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card DJ, Hermanowski-Vosatka A, Bergstrom JD, Sparrow CP, Detmers PA, Chao YS. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole JE, Mitra AT, Monaco C. Treating atherosclerosis: the potential of Toll-like receptors as therapeutic targets. Expert Rev Cardiovasc Ther. 2010;8:1619–1635. doi: 10.1586/erc.10.149. [DOI] [PubMed] [Google Scholar]
- 52.Leon CG, Tory R, Jia J, Sivak O, Wasan KM. Discovery and development of toll-like receptor 4 (TLR4) antagonists: a new paradigm for treating sepsis and other diseases. Pharm Res. 2008;25:1751–1761. doi: 10.1007/s11095-008-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]