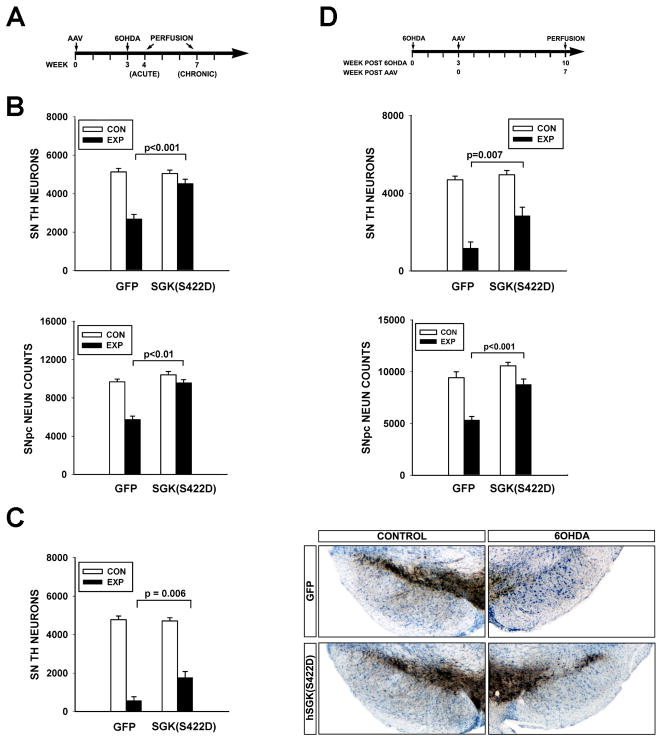
Figure 3. Constitutively active SGK1 protects SN dopaminergic neurons from neurotoxin-induced cell death.
A, Mice received a unilateral intra-nigral injection of AAV hSGK1(S422D) at Time = 0, followed in 3 weeks by an intra-striatal injection, on the same side, of 6-OHDA. B, At one week post-lesion, there was a greater loss of SN TH-positive neurons in mice receiving an injection control of AAV GFP (N=7) in comparison to mice treated with AAV hSGK1(S422D) (N=6) (p<0.001, ANOVA; Tukey post-hoc as shown) (CON: Control, uninjected side; EXP: Experimental, injected side). Similarly, there was a greater loss of NeuN-positive profiles in mice treated with AAV GFP (N=7) than in the mice treated with AAV hSGK1(S422D) (N=6) (p<0.001 ANOVA; Tukey post-hoc as shown). C, Survival was assessed at 4 weeks post-lesion, and the protective effect of AAV hSGK1(S422D) (N=5) was still evident in comparison to the AAV GFP injection control group (N=6) (p<0.001, ANOVA; Tukey post-hoc as shown). The panels to the right, representative sections immunostained for TH and thionin counter-stained, illustrate this protective effect. D, AAVs were injected at 3 weeks after 6-OHDA, and then neuron survival assessed at 7 weeks after AAV. In this paradigm, while there was a 75% loss of neurons in the GFP condition, there was only a 43% loss in the hSGK1(S422D) condition (p<0.001, ANOVA; Tukey post-hoc as shown; AAV GFP N=6, AAV hSGK1(S422D) N=7). Again, improved survival, rather than just preservation of phenotype, was confirmed by counts of NeuN-positive profiles (p<0.001, ANOVA; Tukey post-hoc as shown).