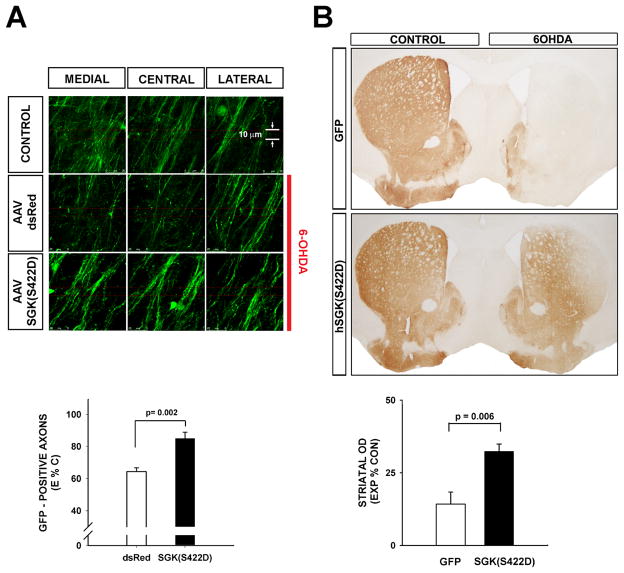
Figure 4. Constitutively active SGK1 protects dopaminergic axons from acute neurotoxin-induced retrograde degeneration.
A, TH-GFP mice received an intra-nigral injection of AAV hSGK1(S422D) or AAV dsRed as a control, followed in 3 weeks by an intra-striatal injection of 6-OHDA. At 7 days post-lesion, the number of surviving dopaminergic axons in the MFB was determined by confocal optical sectioning. Each individual panel represents a maximal projection of twenty 0.1 μm optical sections. For each side of the brain, three adjacent maximal projections are shown for the medial, central, and lateral MFB. It can be seen in these representative images that 6-OHDA induces a loss of axons in the mice that received AAV dsRed in comparison to the CONTROL, contralateral, non-6-OHDA-injected side. However, axons are preserved in mice that received AAV hSGK1(S422D). This effect is shown quantitatively in the graph in which the number of axons remaining on the lesioned (Experimental (E)) side of the brain is expressed as a percent of the contralateral, non-lesioned Control (C) (E%C). A significant protective effect on axons is observed (p=0.002, t-test; AAV dsRed N=5, AAV hSGK1(S422D) N=6). B, in a separate experiment mice were sacrificed at 4 weeks post-lesion and processed for TH immunoperoxidase staining of the striatum. The AAV hSGK1(S422D)-treated mice demonstrated a significant preservation of striatal dopaminergic TH-positive staining, as shown in the representative coronal sections and by quantitative determination of optical densities as shown in the graph (p = 0.006, t-test; AAV GFP N=6, AAV hSGK1(S422D) N=5) (CONTROL: contralateral, uninjected side).