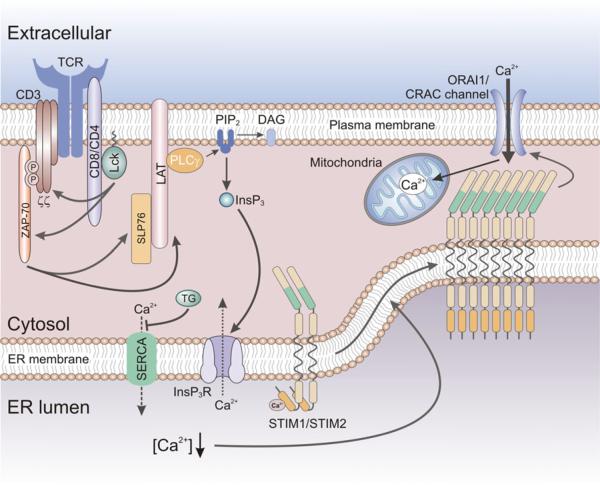
Fig. 1.
Store-operated Ca2+ entry (SOCE) in T cells. Antigen binding to the T cell receptor (TCR) results in the activation of protein tyrosine kinases Lck and ZAP-70, phosphorylation of signaling molecules LAT and SLP-76, and the activation of phospholipase C (PLC) γ. PLCγ catalyzes the hydrolysis of PIP2 into the second messengers InsP3 and DAG. InsP3 gates InsP3 receptors in the membrane of the ER resulting in the (active) release of Ca2+ from ER Ca2+ stores. Passive depletion of ER Ca2+ stores can be achieved by the inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump by thapsigargin (TG). The reduction in [Ca2+]ER resulting from active or passive store depletion is sensed by STIM1 and its homologue STIM2, resulting in their oligomerization and translocation to ER-plasma membrane (PM) junctions. There STIM1 and STIM2 bind to and open CRAC channels formed by ORAI proteins located in the plasma membrane, resulting in store-operated Ca2+ entry (SOCE). SOCE is sustained by Ca2+ uptake into mitochondria that are located close to the plasma membrane, thereby preventing Ca2+-dependent inactivation of CRAC channel function. DAG diacylglycerol, InsP3 inositol 1,4,5-trisphosphate, LAT linker of activated T cells, Lck lymphocyte-specific protein tyrosine kinase, PIP2 phosphatidylinositol 4,5-bisphosphate, SHP-76 SH2 domain containing leukocyte protein of 76 kDa, ZAP-70 Zeta-chain-associated protein kinase 70