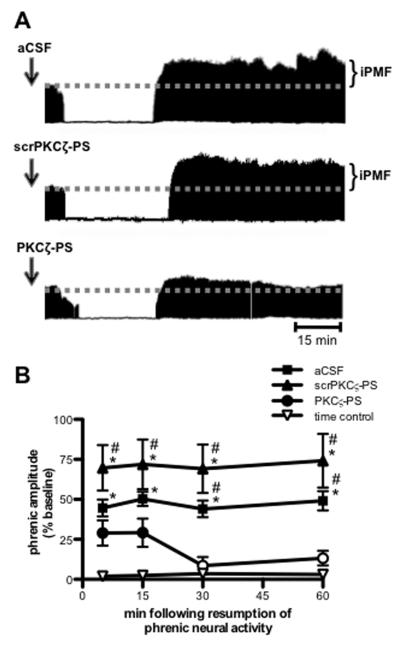
Figure 1.
Spinal aPKC activity is necessary for iPMF. A. Representative compressed and integrated phrenic neurograms before, during and for 60 min following a 30 min neural apnea, illustrating that rats receiving injections (arrows) of intrathecal aCSF (top) or scrPKCζ-PS (middle) prior to neural apnea expressed a prolonged increase in phrenic burst amplitude following resumption of respiratory neural activity, indicating iPMF. Only modest, transient increases in phrenic burst amplitude post-neural apnea were observed rats receiving intrathecal PKCζ-PS (bottom) prior to a neural apnea, indicating that spinal aPKC inhibition attenuated and shortened iPMF. B. Average change in phrenic burst amplitude from baseline for 60 min following resumption of respiratory neural activity in rats receiving intrathecal aCSF (squares), scrPKCζ-PS (triangles) or PKCζ-PS (circles) 20 min prior to neural apnea. Prolonged iPMF is expressed in rats receiving control injections of aCSF or scrPKCζ-PS, when compared to baseline or time controls (inverted triangles). Rats receiving intrathecal PKCζ-PS (circles) expressed only transient, modest iPMF since phrenic burst amplitude was significantly increased from baseline only at 5 and 15 min following resumption of respiratory neural activity, which was not significantly different from time controls. No changes in phrenic burst amplitude were observed in time controls receiving similar surgery, but no neural apnea. Mean values + SEM. Filled symbols indicate significantly different than baseline; *significantly different from time controls; # significantly different from PKCζ-PS rats; p<0.05.