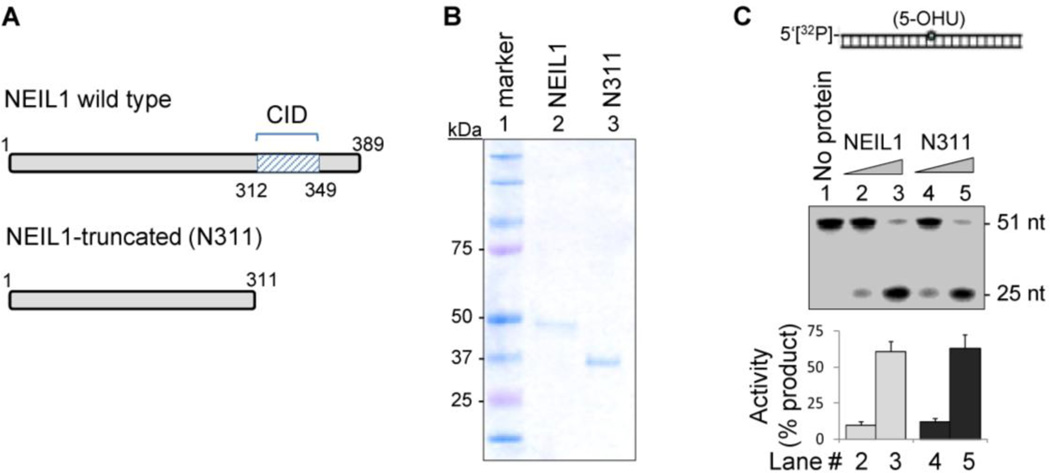
Figure 2.
NEIL1’s common interaction domain (CID)-containing C-terminus is dispensable for DNA glycosylase activity in vitro. Recombinant wild-type (WT) and truncated (N311) mutant of NEIL1 (A; Coomassie-stained gel in B) show similar DNA glycosylase/AP lyase activity with a 5-OHU-containing 5'-32P-labeled 51-mer oligonucleotide duplex substrate to produce 25 nt oligo (C). Lanes 2 and 3: 10 and 50 fmol WT NEIL1; lanes 4 and 5: 10 and 50 fmol N311 mutant.