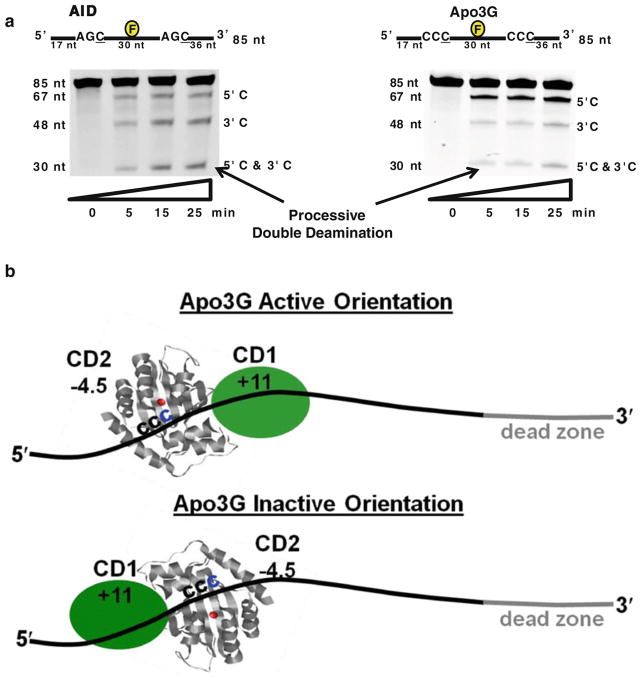
Fig. 4.
Model for Apo3G deamination polarity. a A Fluorescein (F)-labeled 85-nt ssDNA containing 2 identical deamination motifs (AGC for AID, left and CCC for Apo3G, right) was incubated with AID or Apo3G. Following treatment with Uracil-DNA Glycosylase and hot alkali, single deaminations at the 5′ site (5′C) or the 3′ site (3′C) are detected as the appearance of the labeled 67-nt or 48-nt fragment, respectively. Processive deamination at both the 5′ and 3′ sites (5′C and 3′C) on the same substrate is detected as the presence of a 30-nt fragment. AID deaminates 5′ and 3′ target motifs with equal efficiency (left gel), whereas Apo3G deaminatates the 5′ target motif preferentially (right gel). b Proposed model for Apo3G deamination polarity. Apo3G can bind ssDNA, with equal probability, in active or inactive orientations. In the active orientation, the active domain CD2 (charge = −4.5) is positioned toward the 5′-end and CD1 (+11) is facing the 3′-end. In the inactive orientation, Apo3G residues responsible for substrate specificity are distant from the 5′-CCC target motif and away from zinc ion, thereby precluding catalysis. The inability of the CD2 domain to bind to 30 nt at the 3′ end in the active orientation results in a “deamination dead zone”