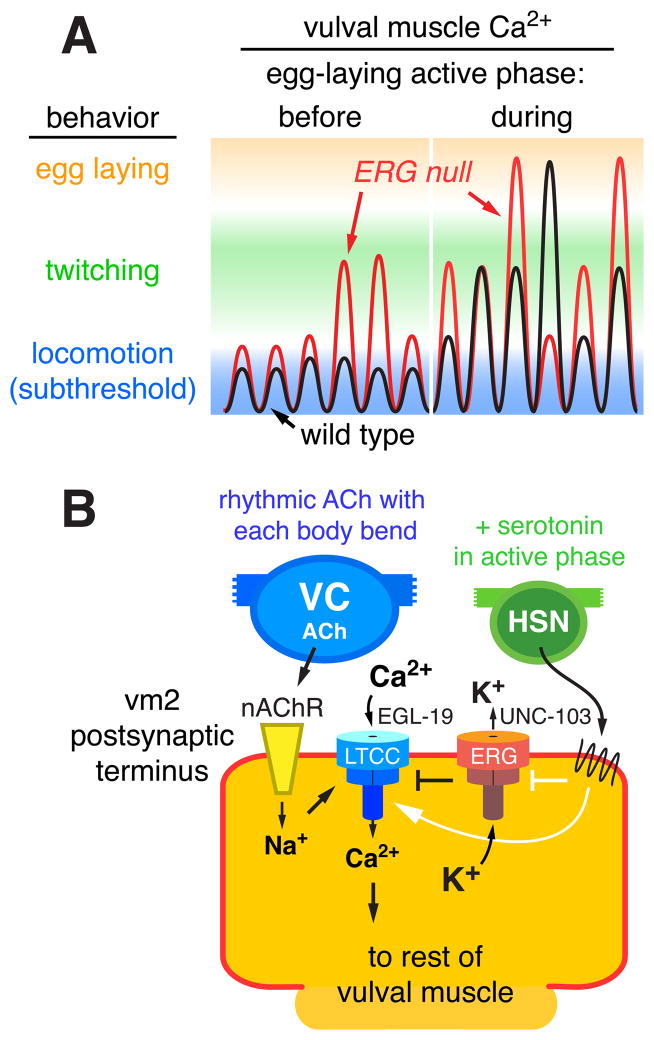
Figure 9. Working model: ERG limits postsynaptic Ca2+ transients and behavioral responses to rhythmic synaptic excitation.
(A) Schematic of vulval muscle Ca2+ signals on a log scale during six body bends before and six more body bends during the active phase of egg laying. Before the active phase, excitatory postsynaptic potentials are subthreshold and do not trigger detectable vulval muscle Ca2+ transients or contraction. During the active phase, increased postsynaptic electrical excitability increases the probability of Ca2+ transients that drive vulval muscle twitching (green) or large transients that drive complete vulval muscle contraction and egg laying (orange). In the ERG null mutant (red trace), elevated postsynaptic excitability during both the inactive and active phases increases the probability of super-threshold responses that trigger Ca2+ transients.
(B) Schematic of control of synaptic excitation at the vulval muscles. ACh release from the VC motor neurons, occurring rhythmically at a particular phase of each locomotor body bend, activates nAChR on the vm2 postsynaptic terminus. The resulting local membrane depolarization from Na+ influx activates EGL-19 voltage-gated L-type Ca2+ channels (LTCC) and plasma membrane Ca2+ entry. UNC-103 ERG K+ channels localize to and promote repolarization of the postsynaptic terminus to antagonize LTCC activity and keep postsynaptic Ca2+ levels at a subthreshold level that fails to trigger vulval muscle contraction. Loss of ERG leads to sustained Ca2+ entry through LTCC. The HSN motor neuron releases serotonin that acts through G protein coupled receptors to increase vulval muscle response to ACh and promote the active phase of egg-laying behavior. Solid white arrows and bar indicate possible targets of serotonin signaling: activation of Ca2+ signaling through LTCC and/or inhibition of ERG.