Scheme 2. Synthesis of compounds 41–48.a.
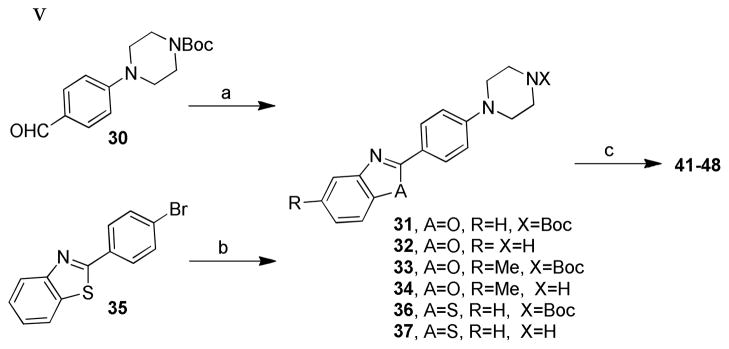
aReaction conditions: (a) (i) 2-aminophenol or 2-amino-4-methylphenol, O2, TEMPO, toluene, reflux overnight, 23– 81%; (ii) TFA/DCM, RT, 3 h, 66–77%; (b) (i) tert-butyl piperazinecarboxylate/tBuOK, Pd2(dba)3 (5% mmol), BINAP (15% mmol), toluene, reflux, 12 h, 60%; (ii) TFA/DCM, 25 °C, 3 h, 85%; (c) RCHO/HCOOH (2 equiv), anhydrous DMF, 100 °C, 3 h, 6–44%.
