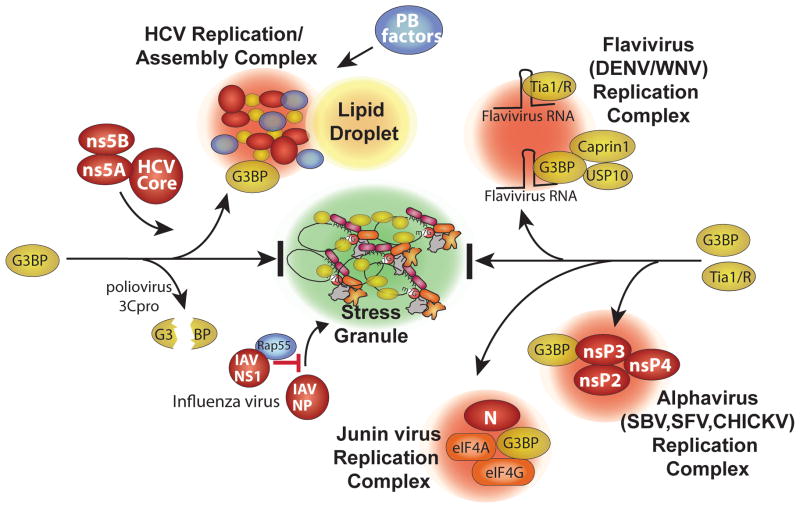
Fig. 2.
Virus blockade and co-opting of stress granule responses. Specific points/proteins where viruses interact with and inhibit or divert the RNA granule assembly pathway are shown. Poliovirus 3C proteinase cleaves the critical SG nucleating protein G3BP1. Several viruses co-opt G3BP and divert it into novel virus induced foci. HCV diverts G3BP1 into replication/assembly complexes together with HCV core, ns5A and ns5B proteins that also associate with lipid droplets. HCV complexes also contain many P-body components detailed in Fig 3. Flaviviruses divert G3BP1 (with USP10 and caprin1) and TIA1/TIAR to replication complexes by binding the host proteins on virus RNAs. Alphaviruses recruit G3BP1 into viral replication complexes via direct interaction viral protein nsP3. Junin virus (possibly N and G proteins) recruits G3BP1 into replication complexes that also contain translation factors eIF4G and eIF4A.