Figure 1.
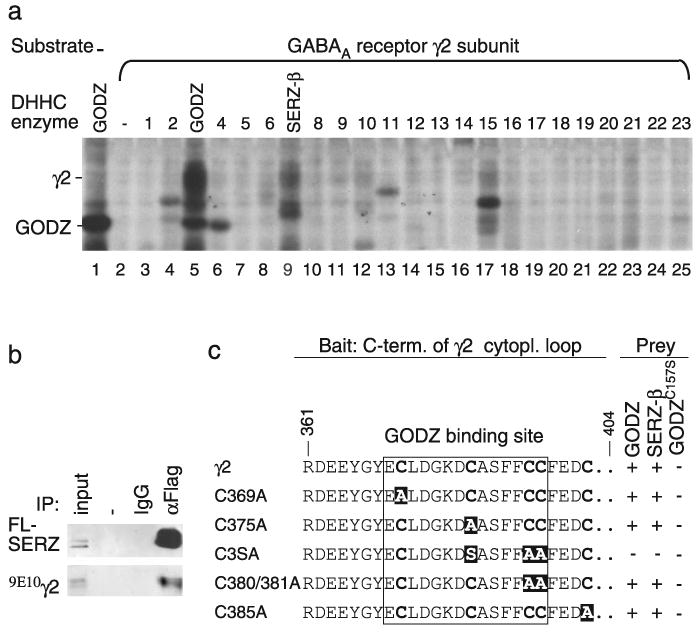
GODZ and SERZ-β palmitoylate the γ2 subunit in vitro and interact with indistinguishable sequence specificity. a, Individual DHHC clones (1–23, with DHHC3 and 7 indicated as GODZ and SERZ-β, respectively) were cotransfected with (9E10)γ2 into HEK293T cells. The cells were metabolically labeled with 3H-palmitate and the proteins separated by SDS-PAGE and analyzed by fluorography. Note the selective labeling of the γ2 subunit in cells expressing GODZ (lane 5) or SERZ-β (lane 9), with a trace of labeling also apparent with DHHC15 (lane 17). No labeling above background was evident in the absence of transfected γ2 subunit (lane 1) or in the presence of any of the other DHHC proteins (lanes 2–4, 6–8, 10–25). b, HEK293T cells were cotransfected with the γ2 subunit and FL-SERZ-β and tested for interaction by coimmunoprecipitation and Western blot analysis. Precipitation of FL-SERZ-β was confirmed with mAb anti-FLAG. FLAG rabbit antiserum, but not IgG, resulted in efficient coimmunoprecipitation of the (9E10)γ2 subunit analogous to results previously shown for GODZ (Keller et al., 2004). c, Yeast two-hybrid assays using a γ2 subunit fragment (amino acids 361–404) or the indicated mutant derivatives as bait were used to test for interaction with full-length GODZ, SERZ-β, or GODZC157S as prey. The GODZ binding site (Keller et al., 2004) is boxed with the mutated cysteines highlighted in white on a black background. Note the identical sequence specificity of GODZ and SERZ-β for interaction with the γ2 subunit. Enzymatically inactive GODZC157S failed to interact with the γ2 constructs.
