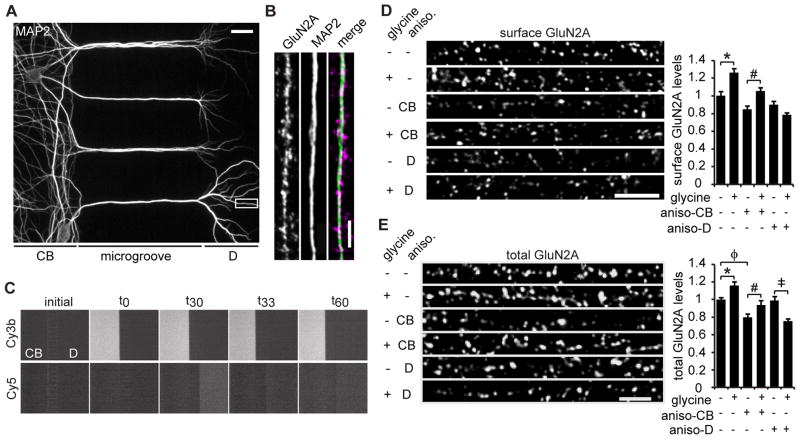
Figure 2. Dendritic protein synthesis is required for glycine-induced insertion of GluN2A.
(A) MAP2 staining of 25 DIV neurons cultured in a microfluidic device (CB: cell bodies, D: dendrites). Scale bar is 20 μm. (B) A representative dendritic region immunostained for MAP2 and GluN2A. Scale bar is 3 μm. (C) Time-lapse imaging shows microfluidic chambers maintain fluidic isolation throughout the glycine treatment paradigm. Microfluidic devices were filled with PBS (initial), then left-side PBS was replaced with PBS plus Cy3b dye (t0), and, 30 min later, right-side PBS was replaced with PBS plus Cy5 dye (t30). After 3 min, PBS plus Cy5 dye was replaced with PBS alone (t33) and allowed to sit for an additional 30 min (t60). The Cy3 and Cy5 dye solutions remained restricted to the cell body (CB) and dendrite (sides), respectively, for the duration of the experiment. (D) Hippocampal neurons were cultured in microfluidic devices. Anisomycin was applied to either the cell body or the dendrite compartment for 30 min, followed by glycine or vehicle application to the dendrite compartment for 3 min, and an additional 30 min of incubation in solution without glycine. Anisomycin or DMSO was present in all solutions. Then, the neurons were fixed and immunostained for surface GluN2A protein (ANOVA, Bonferroni t-tests; n = 65; * p < 0.001, # p = 0.008). Scale bar is 5 μm. (E) Neurons were treated as in (D), except that total GluN2A was immunostained (ANOVA, Bonferroni t-tests; n = 65; * p = 0.003, # p = 0.011, ‡ p < 0.001, φp = 0.004). Scale bar is 3 μm. Data are mean ± s.e.m.