Abstract
The arrival of new antiviral drugs to treat chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections has given rise to great expectations along with concerns regarding the selection of drug-resistant variants. Many lessons learned from HIV therapeutics can be helpful for designing adequate treatment strategies against viral hepatitis, the avoidance of sequential weak monotherapies being one of them. While HIV, HBV and HCV share many biological features, including very rapid viral dynamics, distinctive characteristics explain why the speed of selection of drug resistance differs substantially between these viruses, being faster for HCV than for HIV, and slower for HBV.
Chronic infection due to HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV) accounts for a substantial proportion of deaths worldwide. Around 36 million people are currently living with HIV. These numbers are approaching 400 million and 200 million for chronic HBV and HCV infections, respectively. Because of similar routes of transmission, these viruses are seen more frequently than expected in co-infection [1,2]. Besides sharing epidemiological niches, HIV, HBV and HCV share several biological similarities, which largely explain the therapeutic difficulties arising when treating any of them, drug resistance being one if not the most challenging complication.
Viral dynamics are rapid for all three of these viruses. Estimates of the daily production of virions are in the range of 1010 for HIV [3], 1012 for HCV [4] and 1012-1013 for HBV [5,6] The half-life of free viral particles is very short, below 1 hour for HIV [7], and between 2-3 hours for HCV [4,8]. For HBV there is some controversy and estimates vary between 3 and 24 hours [5,6]. What is more different is the half-life of infected cells. It has been estimated to be about 1 day for CD4+ T lymphocytes productively infected with HIV [9], several days or weeks for hepatocytes infected with HCV [4] and up to 100 days for those infected with HBV, with large lifespan heterogeneity [10].
Mutations occur frequently during the replication of HIV, HBV and HCV. The reverse transcriptase enzymes of HIV and HBV as well as the RNA dependent RNA polymerase of HCV are intrinsically error prone and lack proofreading function, allowing for frequent replication errors to occur. The result is the generation of multiple viral variants, known as a quasispecies, that coexist and reach population densities in direct proportion to their relative replication fitnesses. It has been predicted that every nucleoside of the 3.2 Kb HBV genome [6] or the 10 Kb HIV [11] and HCV genomes theoretically can be substituted every day within a given infected patient. Table 1 summarises the main distinctive viral dynamic features of these three viruses.
Table 1.
Main distinctive viral dynamic features of HIV, HBV and HCV.
| HIV | HBV | HCV | |
|---|---|---|---|
| Virus | |||
| • Daily production of virions per day | 1010 | 1012-1013 | 1012 |
| • Half-life of free virions (hours) | 1 | 3-24 | 2-3 |
| • Half-life of intracellular virions | Days (dependent of infected cells t1/2) | Months (dependent of infected cells t1/2) | Hours (non-dependent of infected cells t1/2) |
| • Mutation rate | Very high | High | Very high |
| • Constraints due to ORFs in targeted viral enzymes | Moderate | High | None |
| • Immune mediated escape mutants | Frequent | Unfrequent | Frequent |
|
| |||
| Target cells | |||
| • Half-life of infected cells | Days | Months | Weeks |
| • Size of susceptible cells compartment | Large | Small | Probably large |
| • Intracellular viral reservoir | Yes | Yes | No |
| (integrated cDNA) | (cccDNA) | ||
ORFs, overlapping reading frames; cDNA, complementary DNA; cccDNA, covalently closed circular DNA
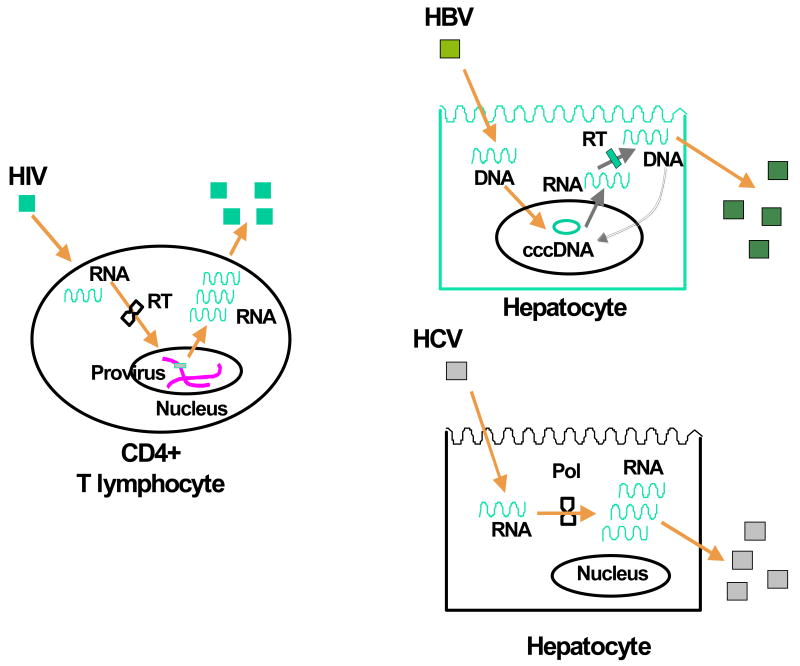
Since any drug pressure may act to select pre-existing drug resistance viral variants, the speed for selecting drug resistance mainly depends of the turnover of the viral nucleic acid acting as source of new viral genomes. In the case of HIV, it is the proviral DNA integrated within the chromosomes of infected cells. For HBV, it is the cccDNA present within the nucleus of infected hepatocytes as extra-chromosomal (episomal) material. Finally, for HCV there is no stable reservoir of genetic material and the HCV-RNA strands present in infected hepatocytes serve as templates for producing new HCV virions that soon thereafter are released (Figure 1).
Figure 1.
Schematic representation of the virus life cycle for HIV, HBV and HCV.
The viral genetic material within infected cells is relatively stable and shows longer half-life for HIV and HBV, in comparison with HCV. Whereas HIV proviral DNA may persist as long as the lifespan of an infected CD4+ T lymphocyte, and the same applies to HBV cccDNA within infected hepatocytes [12,13], HCV-RNA strands are short-lived molecules with a half-life of about 10 hours [14], in constant renewal replicating in cytoplasmic vesicular membraneous structures within infected hepatocytes [15]. Given these facts, it is easy to understand that the time needed for selecting drug resistance mutations, present at baseline only as minority genomic variants, which then expand and fill a major part of the virus population should be longer for HBV than for HIV, and that it must be particularly short for HCV. This may explain in part why resistance to lamivudine used as monotherapy may be recognised within three weeks in HIV [16], while it only develops after several months to years of therapy in HBV [17].
The major determinants involved in the selection of drug-resistant mutants for all these viruses are the fitness of the mutants and the replication space available for the spread of mutants [18]. In chronic hepatitis B, the replication space is provided by hepatocyte turnover, which allows the loss of HBV wild-type infected cells and the generation of non-infected hepatocytes that are susceptible to new HBV mutant infections. This process is usually very slow in chronic hepatitis B because the immune mediated killing of infected cells is slow [19]. By contrast, in HIV, the turnover of CD4+ T lymphocytes is quite rapid allowing the mutant viruses to expand rapidly. The spread of mutants in the presence of the drug will also depends on the relative fitness of these variants. For HCV, the diversity of the viral genome is greater than for HBV, and the proportion of infected hepatocytes and the rate of superinfection of these cells is not well known, but the extraordinary rapidity of emergence of drug-resistant HCV mutants is in agreement with the short turnover of HCV-RNA molecules in the cytosol of infected hepatocytes.
In a situation in which most potential target cells are already infected and releasing virions, it is clear that infected cells with a long half-life will provide only a minimal opportunity for replacing the original virus population by a new one of drug-resistant variants. This is the case for HBV, whose infected hepatocytes may survive for several weeks or months [10,20]. In contrast, CD4+ T lymphocytes infected with HIV show a shorter half-life (∼1 day) [9]. This is why the dynamics of selection of drug resistance are so different comparing HBV and HIV, despite their respective genetic material being archived within the nucleus of infected cells.
Besides the different half-life of each of the respective viral genetic materials, other factors may explain the relatively slow selection of drug resistance in HBV compared to HIV and/or HCV (Table 2). Among others are the constraints imposed by the fact that the HBV genome shows overlapping reading frames. In this way, changes at one position may affect the structure and function of more than one viral protein. Indeed, it is well known that some lamivudine-associated resistance mutations may modify the antigenicity of the HBV surface antigen, as a result of the large overlap between the HBV polymerase and envelope genes [21]. HBV escape mutants induced by antiviral therapy have recently attracted much attention as they may represent a public health threat in the near future [22]. Moreover, mutants of the viral polymerase gene may induce mutations in the overlapping surface antigen which may then generate defective or less infectious mutants, that may need trans-complementation of the mutant protein by wild type to package and propagate the mutant virus (some of the M204I and the A181T mutants are examples) [23].
Table 2.
Factors explaining the slower selection of drug resistance in HBV compared to HIV and HCV.
|
Another mechanism by which drug resistance in HBV may be selected for much slowly than in HIV or HCV depends on the immune system. Rapid selection of immune escape mutants has been described for HIV and HCV [24,25], while some level of immune tolerance may persist throughout the entire course of chronic HBV infection [26], providing little selective pressure.
Several therapeutic consequences derive from these biological considerations. The first is that eradication of HIV and HBV will not be attainable even after several years of complete virus suppression with current antiviral therapies, since relatively stable reservoirs of genetic viral material may exist awaiting to be awaken in the advent drug pressure is discontinued. In contrast, the fragile nature of HCV-RNA molecules in continuous turnover may provide a unique opportunity for eradication. Indeed, the vast majority of patients who achieve sustained virological response with interferon-based therapies do not show a rebound in HCV replication thereafter [27,28], suggesting that the virus has definitively been eliminated. This is true even in HIV-HCV co-infected persons, in whom immunodeficiency might rise suspicion of possible late relapses [29,30].
A last therapeutic implication of these differences in the kinetics of selection of drug-resistant mutants is the difficulty to prove the benefit of combination therapy in chronic hepatitis B. It was relatively easy to demonstrate this benefit against HIV and it is currently being shown against HCV using STAT-C molecules [31]. Although drugs targeting different steps of the life cycle of both HIV and HCV have been developed, and this has not been the case for HBV, it is clear that the relatively slow rate of emergence of drug-resistant HBV mutants in comparison with HIV or HCV keeps open the possibility of continuing the use of monotherapy against HBV. Clearly, it will not be the case using drugs with relatively low potency, suboptimal dosing and/or low genetic barrier for resistance, such as lamivudine, emtricitabine, telbivudine or adefovir. However, it may apply to drugs such as entecavir or tenofovir, which have much more potent antiviral activity and a high genetic barrier to resistance, and for which the annual rate of selection of resistance is below 1-2%, at least in drug-naïve chronic hepatitis B patients [32-34].
Acknowledgments
Funding: This work was supported in part by grants from Fundación IES (Investigación & Educación en SIDA), Agencia Laín Entralgo, Red de Investigacion en SIDA (RIS, project ISCIII-RETIC RD06/006) the European Commission VIRGIL and NEAT projects, and NIH grants RR06555, AI065256 and AI28433. Also, portions of this work were done under the auspices of the U. S. Department of Energy under contract DE-AC52-06NA25396. We would like to thank Pablo Barreiro for helpful comments.
References
- 1.Soriano V, Puoti M, Bonacini M, et al. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV international panel. AIDS. 2005;19:221–240. [PubMed] [Google Scholar]
- 2.Soriano V, Puoti M, Sulkowski M, et al. Care of patients coinfected with HIV and hepatitis C virus: 2007 updated recommendations from the HCV-HIV International Panel. AIDS. 2007;21:1073–1089. doi: 10.1097/QAD.0b013e3281084e4d. [DOI] [PubMed] [Google Scholar]
- 3.Perelson A, Neumann A, Markowitz M, Leonard J, Ho D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 4.Neumann A, Lam N, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon alpha therapy. Science. 1998;282:103–7. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 5.Tsiang M, Rooney J, Toole J, Gibbs C. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology. 1999;29:1863–9. doi: 10.1002/hep.510290626. [DOI] [PubMed] [Google Scholar]
- 6.Murray J, Purcell R, Wieland S. The half-life of hepatitis B virions. Hepatology. 2006;44:1117–21. doi: 10.1002/hep.21364. [DOI] [PubMed] [Google Scholar]
- 7.Ramratnam B, Bonhoeffer S, Binley J, et al. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet. 1999;354:1782–5. doi: 10.1016/S0140-6736(99)02035-8. [DOI] [PubMed] [Google Scholar]
- 8.Neumann A, Lam N, Dahari H, et al. Differences in viral dynamics between genotypes 1 and 2 of hepatitis C virus. J Infect Dis. 2000;182:28–35. doi: 10.1086/315661. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz M, Louis M, Hurley A, et al. A novel antiviral intervention results in more accurate assessment of HIV-1 replication dynamics and T-cell decay in vivo. J Virol. 2003;77:5037–8. doi: 10.1128/JVI.77.8.5037-5038.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak M, Bonhoeffer S, Hill A, Boehme R, Thomas H, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci USA. 1996;93:4398–402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perelson A, Essunger P, Ho D. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS. 1997;11 A:17–24. [PubMed] [Google Scholar]
- 12.Moraleda G, Saputelli J, Aldrich G, Averett D, Condreay L, Mason W. Lack of effect of antiviral therapy in non-dividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–8. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Bichko V, Seeger C. Effect of alpha interferon on the hepatitis C virus replicon. J Virol. 2001;75:8516–23. doi: 10.1128/JVI.75.18.8516-8523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahari H, Ribeiro R, Rice C, Perelson A. Mathematical modeling of subgenomic hepatitis C virus replication in Huh-7 cells. J Virol. 2007;81:750–60. doi: 10.1128/JVI.01304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tisdale M, Kemp S, Parry N, Larder B. Rapid in vitro selection of HIV type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–6. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai C, Chien R, Leung N, et al. A one year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61–8. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 18.Litwin S, Toll E, Jilbert A, Mason W. The competing roles of virus replication and hepatocyte death rates in the emergence of drug-resistant mutants: theoretical considerations. J Clin Virol. 2005;34 1:96–107. doi: 10.1016/s1386-6532(05)80018-6. [DOI] [PubMed] [Google Scholar]
- 19.Summers J, Jilbert A, Yang W, et al. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc Natl Acad Sci USA. 2003;100:11652–9. doi: 10.1073/pnas.1635109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewin S, Ribeiro R, Walters T, et al. Analysis of hepatitis B viral load decline under potent therapy: complex decay profiles observed. Hepatology. 2001;34:1012–20. doi: 10.1053/jhep.2001.28509. [DOI] [PubMed] [Google Scholar]
- 21.Locarnini S. Molecular virology and the development of resistant mutants: implications for therapy. Semin Liver Dis. 2005;25 1:9–19. doi: 10.1055/s-2005-915645. [DOI] [PubMed] [Google Scholar]
- 22.Sheldon J, Soriano V. Hepatitis B virus escape mutants induced by antiviral therapy. J Antimicrob Chemother. 2008;61:766–8. doi: 10.1093/jac/dkn014. [DOI] [PubMed] [Google Scholar]
- 23.Villet S, Pichoud C, Billioud G, Trepo C, Zoulim F. Impact of hepatitis B virus rt181V/T mutants on hepatitis B treatment failure. J Hepatol. 2008;48:747–55. doi: 10.1016/j.jhep.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Petravic J, Loh L, Kent S, Davenport M. CD4+ target cell availability determines the dynamics of immune escape and reversion in vivo. J Virol. 2008;82:4091–101. doi: 10.1128/JVI.02552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuntzen T, Timm J, Berical A, et al. Viral sequence evolution in acute hepatitis C virus infection. J Virol. 2007;81:11658–68. doi: 10.1128/JVI.00995-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau G, Cooksley H, Ribeiro R, et al. Impact of early viral kinetics on T-cell reactivity during antiviral therapy in chronic hepatitis B. Antivir Ther. 2007;12:705–18. [PubMed] [Google Scholar]
- 27.Torres-Ibarra R, Cano-Dominguez C. Sustained virological response after more than 3 years of the end of treatment with conventional alpha-2b or pegylated alpha-2a interferon. J Hepatol. 2007;46 1:247. [Google Scholar]
- 28.Maylin S, Martinot-Peignoux M, Boyer N, et al. Sustained virological response is associated with eradication of HCV and decrease in anti-HCV titer in patients treated for chronic hepatitis C with interferon alpha-2b or pegylated interferon alpha-2b plus ribavirin. Hepatology. 2007;46 1:343–4. [Google Scholar]
- 29.Soriano V, Maida I, Nuñez M, et al. Long-term follow-up of HIV-infected patients with chronic hepatitis C virus infection treated with interferon-based therapies. Antivir Ther. 2004;9:987–92. doi: 10.1177/135965350400900616. [DOI] [PubMed] [Google Scholar]
- 30.Berenguer J, Alvarez-Pellicer J, Lopez-Aldeguer J, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in HIV/HCV co-infected patients. 15th CROI; Boston. February 3-6; 2008. Abstract 60. [Google Scholar]
- 31.Soriano V, Madejon A, Vispo E, et al. Emerging drugs for hepatitis C. Expert Opin Emerg Drugs. 2008;13:1–19. doi: 10.1517/14728214.13.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Gish R, Lok A, Chang T, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–44. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Tan J, Degertekin B, Wong S, Husain M, Oberhelman K, Lok A. Tenofovir monotherapy is effective in hepatitis B patients with antiviral treatment failure to adefovir in the absence of adefovir-resistant mutations. J Hepatol. 2008;48:391–8. doi: 10.1016/j.jhep.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Reijnders J, Janssen H. Potency of tenofovir in chronic hepatitis B: mono or combination therapy? J Hepatol. 2008;48:383–386. doi: 10.1016/j.jhep.2007.12.006. [DOI] [PubMed] [Google Scholar]