Abstract
Trace memories are formed when a stimulus event becomes associated with another event that occurs later in time and is discontinuous with the first event. The formation of trace memories enhances the survival of newly generated neurons in the dentate gyrus of the adult hippocampus (Gould etal., 1999a). Here we tested whether the acquisition of trace memories early during training is sufficient to enhance cell survival. We also examined whether the new neurons affected by trace memory formation persist indefinitely or only as long as the hippocampus is necessary for the expression of those memories. Groups of adult rats were injected with bromodeoxyuridine (BrdU), a marker of dividing cells, and trained 1 week later with paired stimuli using a trace eyeblink conditioning task or exposed to the same number of unpaired stimuli. Cell survival was assessed after different numbers of training trials and survival periods after training. Overall cell survival was not enhanced by exposure to 200 trials of paired stimuli during trace conditioning. However, there was a positive correlation between performance of individual animals and cell survival. In addition, exposure to 800 trials of paired stimuli during trace conditioning increased the number of BrdU-labeled cells 60 d after training. The vast majority of these cells were neurons and coex-pressed the neuronal markers class III β-tubulin or neuronal nuclei. These data suggest that individual differences in associative learning predict whether new neurons will survive and that once affected, these neurons remain for months and beyond the time when they are required for the retention of trace memories.
Keywords: neurogenesis, trace, eyeblink conditioning, dentate gyrus, BrdU, cell death, individual differences, stress
Introduction
The dentate gyrus of the hippocampus produces new granule neurons throughout adulthood (Altman and Das, 1965; Kaplan and Hinds, 1977). The number of new cells generated in the dentate gyrus of adult rats is significant, estimated at thousands per day (Cameron and McKay, 2001), and most differentiate into neurons (Hastings and Gould, 1999; Markakis and Gage, 1999; van Praag et al., 2002). However, a large percentage of the new neurons degenerate within a few weeks under standard laboratory conditions (Cameron et al., 1993; Dayer et al., 2003). In a previous study, we found that a significant number of the newly generated neurons could be rescued from death after exposure to hippocampal-dependent learning tasks (Gould et al., 1999a). In the experiment, newborn neurons were labeled with bromode-oxyuridine (BrdU), a marker of dividing cells. One week later, at a time when many of these cells die (Cameron et al., 1993; Dayer et al., 2003), groups of animals were either trained on various learning tasks or not trained. One of the tasks, known as trace eyeblink conditioning, involves associating an auditory stimulus with electrical stimulation of the eyelid that occurs later in time and is discontinuous with the auditory stimulus. After exposure to several days of training, animals that had learned the trace conditioning task possessed more BrdU-labeled cells than animals that were naive or trained on tasks that do not require the hippocampus. We also found that newly generated cells may be used in the acquisition of trace memories, at least to the extent that depletion of the population of new neurons resulted in an inability to acquire trace memories but not other types of memories (Shors et al., 2001, 2002).
The hippocampus becomes activated early during trace conditioning and is necessary for acquisition of the learned response (Solomon et al., 1986; McEchron and Disterhoft, 1997). Animals with hippocampal lesions emit virtually no conditioned responses (CRs) during trace conditioning, even after >1000 trials of training (Beylin et al., 2001). However, the hippocampus is only transiently required because lesions of the hippocampus ≥1 week after training do not disrupt the expression of trace memories (Kim et al., 1995; Takehara et al., 2003). Based on these data, we asked the following questions about the effects of trace learning on cell survival. First, does the learning-induced increase in the number of newly generated neurons occur early in training just as the animal is acquiring the association, or does it occur later in training when the animal has demonstrated significant learning? Second, do the new neurons affected by learning persist indefinitely or only as long as the hippocampus is necessary for the expression of those memories? To address these questions, we evaluated the survival of newly generated neurons labeled with BrdU during early acquisition of the trace eyeblink conditioned response and at extended time intervals after learning had occurred.
Materials and Methods
Adult male Sprague Dawley rats (300–400 gm; aged 2–3 months) were individually housed, provided with ad libitum access to food and water, and maintained on a 12 hr light/dark cycle. After at least a 1 week acclimation to the colony room, rats were injected intraperitoneally with BrdU (200 mg/kg). One week later, rats underwent trace eyeblink conditioning with paired stimuli or unpaired training for 200 trials (n = 18) or 800 trials (n = 7). Training with 200 trials was massed within a single day, whereas 800 trials were distributed over 3 consecutive days (300, 300, and 200 per d). Animals were perfused on the 10th day after BrdU injection. The timing of BrdU injections, training, and perfusion was chosen based on a previous study showing that learning enhances the survival of newly born cells that are generated 1 week before training (Gould et al., 1999a). Additional groups trained for 800 trials were killed 30 d (n = 10) or 60 d (n = 9) after the end of training (40 and 70 d after BrdU injection, respectively) (Fig. 1).
Figure 1.
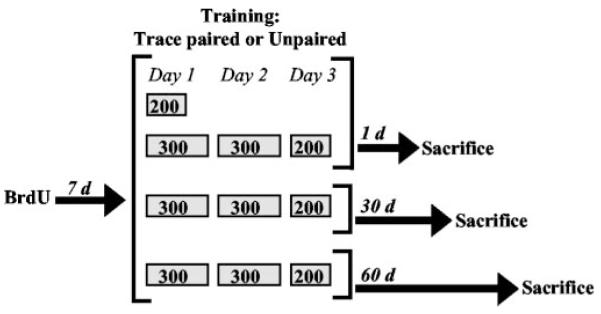
Schematic diagram of experimental design. Rats were injected with BrdU (200 mg/kg, i.p.) and 1 week later underwent 200 or 800 trials of trace eyeblink conditioning with paired stimuli or unpaired training. Two hundred trials of training were massed within 1 d, whereas 800 trials were distributed over 3 consecutive days (300, 300, and 200 per day). Rats were perfused on the 10th day after BrdU injection. Additional group strained for 800 trials were killed 30 or 60 d after training (40 and 70 d after BrdU injection, respectively).
Classical conditioning
Rats were anesthetized with sodium pentobarbital anesthesia (45 mg/kg) supplemented by isoflurane (Baxter Health-care, Deerfield, IL) inhalant. A headstage with four electrodes was secured to the skull. Electrodes consisting of stainless steel wire were implanted subcutaneously to emerge through and around the eyelid. Two electrodes recorded electromyograph (EMG) activity for determination of the eyeblink, and two delivered the periorbital stimulation to elicit the eyeblink reflex. Electrode implantation occurred 2–4 d after BrdU injection.
For eyeblink conditioning, headstages were connected to a cable that allowed free movement within the conditioning chamber. Rats were acclimated to the conditioning apparatus for 1 hr, and spontaneous blink rate was recorded. After 24 hr, rats were exposed to trace conditioning with paired stimuli or unpaired training. During trace conditioning, an 83 dB, 250 msec burst of white noise conditioned stimulus (CS) was separated from a 100 msec, 0.7 mA periorbital shock unconditioned stimulus (US) by a 500 msec trace interval. These stimulus parameters produce learning that is dependent on an intact hippocampus in rats (Beylin et al., 2001). Each block of trace conditioning consisted of 100 trials with every 10 trial sequence composed of one CS-alone presentation, four paired presentations of the CS and US, one US-alone presentation, and four paired presentations of the CS and US. The intertrial interval (ITI) was 25 ± 5 sec. During unpaired training, rats received the same number of CS and US exposures presented in an explicitly unpaired manner. The interstimulus interval and ITI were 10 ± 3 sec. To detect the occurrence of an eyeblink, the maximum EMG response occurring during a 250 msec prestimulus baseline recording period was added to 4× its SD. Responses that exceeded that value and were >3 msec were considered eyeblinks. During trace conditioning, eyeblinks were considered CRs if they began 500 msec before US onset. During exposure to unpaired stimuli, eyeblinks were recorded as responses if they occurred during the same time interval as paired training.
Histology
Brains were processed from animals that were trained with 800 trials and achieved 60% CRs on at least two of the eight training blocks (100 trials each). There were no criteria for animals trained for 200 trials. Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde. Brains were dissected from the skulls, postfixed in 4% paraformaldehyde for 24 hr, and then transferred to PBS. Coronal sections (40 μm) throughout the entire dentate gyrus were cut from half brains on an oscillating tissue slicer. Brain tissue was processed immunohistochemically for BrdU using peroxidase methods. Additional sections were reacted using immunofluorescence methods for BrdU combined with either the cell-type specific marker class III β-tubulin (TuJ1), a marker of immature and mature neurons, or neuronal nuclei (NeuN), a marker of mature neurons. For peroxidase, brain tissue was heated in 0.1 m citric acid, pH 6.0, incubated in 2N HCl and then overnight in primary mouse anti-BrdU (1:250) and 0.5% Tween 20 (1:20), and then incubated for 1 hr in biotinylated anti-mouse antibody (1:200). The sections were then incubated in avidinbiotin–horseradish peroxidase and then in diaminobenzidine. After rinsing in PBS, slides were counterstained with cresyl violet and coverslipped under Permount (Fisher Scientific, Fair Lawn, NJ). For immunofluorescence, the tissue was pretreated in 2N HCl and incubated in primary rat anti-BrdU (1:250; Vector Laboratories, Burlingame, CA) with 0.5% Tween 20 and either mouse anti-NeuN or mouse anti-TuJ1 antibodies (both 1:500; Chemicon, Temecula, CA). Next, the sections were incubated in biotinylated anti-rat antibody (1:250) and incubated with streptavidin Alexa 568 (1:1000; Vector Laboratories) and goat anti-mouse Alexa 488 (1:250; Vector Laboratories) for NeuN or TuJ1.
Slides were coded before quantitative analysis. Stereological analysis of the number of BrdU-labeled cells in the dentate gyrus was conducted on every 12th section of peroxidase-stained tissue throughout the granule cell layer (GCL) and subgranular zone. To avoid counting cell caps, cells in the outermost plane of focus were omitted. For double labeling, the percentage of BrdU-labeled cells in the dentate gyrus that expressed NeuN or TuJ1 was determined by analyzing 25 randomly selected BrdU-labeled cells throughout the GCL and subgranular zone using a Zeiss Axiovert confocal laser scanning microscope (510LSM; Argon and HeNe lasers; Zeiss, Oberkochen, Germany).
Radioimmunoassay of corticosterone
To assess whether differences in cell survival between trace conditioning with paired stimuli or unpaired training are attributable to an effect of stress instead of learning, we trained separate groups of rats on the trace conditioning task with paired (n = 9) or unpaired (n = 9) stimuli for 800 trials. Animals were killed immediately after the last session of training with a group of naive animals that did not receive stimulus exposure (n = 8). Trunk blood was added to test tubes containing 0.1 ml of heparin and centrifuged for 20 min at 3000 rpm. Circulating levels of corticosterone were measured using a solid phase radioimmunoassay system (Coat-A-Count; Diagnostic Products, Los Angeles, CA). Assay sensitivity was 5.7 ng/ml.
Statistical analysis
For analysis of learning data we used ANOVA with repeated measures. The effects of training condition on total cell counts and numbers of double-labeled cells were analyzed using Student’s t tests. ANOVA followed by Newman–Keuls post hoc comparisons was used to analyze the effect of survival time on cell counts and corticosterone concentrations. The relationship between the percentage of conditioned responses and BrdU-labeled cells was evaluated using the Pearson correlation test.
Results
Effects of early acquisition on the number of new cells
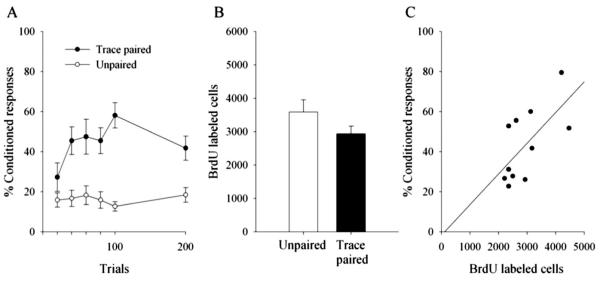
Animals exposed to 200 trials of paired stimuli during trace conditioning emitted more CRs than those that were exposed to 200 trials of unpaired stimuli (F(1,16) = 12.95; p = 0.002) (Fig. 2A). The number of BrdU-labeled cells in the dentate gyrus did not differ in animals exposed to paired versus unpaired stimuli (t(16) =−1.6; p = 0.13) (Fig. 2B). However, there was a positive correlation (r = 0.65; p = 0.03) between the number of BrdU-labeled cells and the percentage of CRs emitted during trace conditioning with paired stimuli (Fig. 2C).
Figure 2.
Early learning in individual animals correlates with cell survival. Although animals exposed to 200 trials of paired stimuli during trace conditioning emitted more conditioned responses than those that were exposed to unpaired stimuli (A), 200 trials of training did not affect the number of BrdU-labeled cells in the dentate gyrus (B). However, there was a positive correlation between the performance of individual animals trained with paired stimuli and survival of new neurons in the dentate gyrus. Those animals that emitted more learned responses possessed more BrdU-labeled cells (C).
Effects of trace conditioning on cell survival 1, 30, and 60 d after training
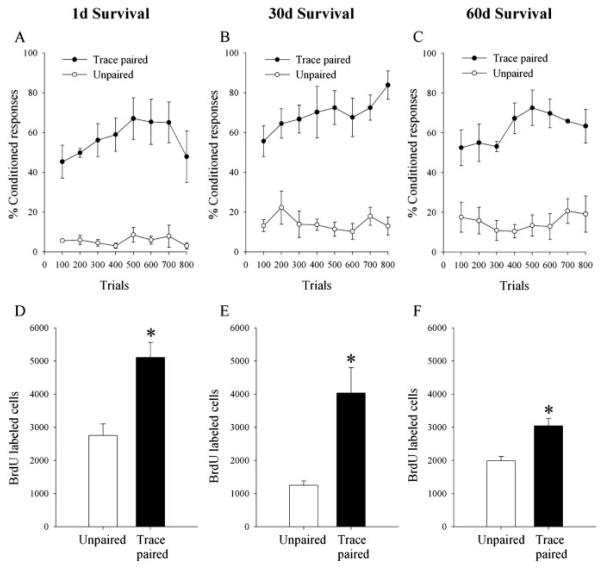
As expected, rats exposed to paired stimuli during trace conditioning emitted more CRs over 800 trials than rats exposed to unpaired training (1 d, F(1,5) = 42.6, p = 0.001; 30 d, F(1,8) = 47.33, p = 0.0001; 60 d, F(1,7) = 34.13, p = 0.0006) (Fig. 3A–C). Exposure to paired stimuli during trace conditioning increased the number of BrdU-labeled cells when compared with exposure to unpaired stimuli and did so at all three time points. That is, exposure to paired stimuli during trace conditioning increased the number of BrdU-labeled cells 1 d (t(5) = 3.89; p = 0.01), 30 d (t(8) = 3.61; p = 0.007), and 60 d (t(7) = 4.27; p = 0.004) after the end of training (Figs. 3D–F, 4).
Figure 3.
Trace memory formation persistently enhances the survival of newly born cells in the dentate gyrus. A–C, Acquisition of the trace eyeblink conditioned response (trace paired) and the unpaired condition. D–F, Total numbers of BrdU-labeledcells in the dentate gyrus of animals 1 d (D), 30 d (E), or 60 d (F) after trace conditioning or unpaired training. Trace conditioning increased the number of BrdU-labeled cells when compared with exposure to unpaired stimuli at all survival times. Regardless of training conditions, the number of cells decreased between 1 and 30 d but not there after. Bars represent mean ±SEM. Significant differences are noted with asterisks.
Figure 4.
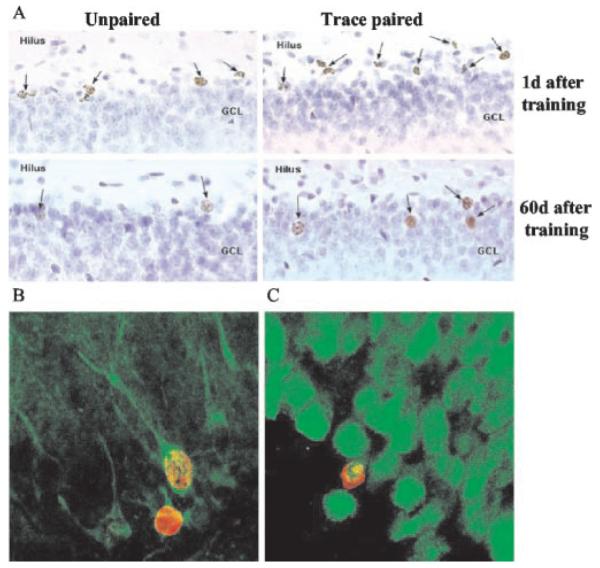
Most of the BrdU-labeled cells differentiate into neurons. Images depict a greater number of BrdU-labeled cells in animals exposed to paired stimuli during trace conditioning than animals exposed to unpaired stimuli at both 1 and 60 d after training (A). Regardless of training conditions, more BrdU-labeled cells are evident at 1 d (10 d after BrdU injection) than at 60 d (70 d after BrdU injection). The majority of BrdU-labeled cells (red) expressed the neuronal markers (green) TuJ1 (B) and NeuN (C).
Although training on the trace conditioning task was associated with an increase in BrdU-labeled cells at the time of perfusion, there was also a decrease in cells that survived over time regardless of training (F(2,20) = 5.75; p = 0.01) (Fig. 3D–F). Animals killed 1 d after the end of training had a greater number of BrdU-labeled cells in the dentate gyrus than those killed at 30 d (p = 0.008) or 60 d (p = 0.01) after training. There was no additional decline in the number of BrdU-labeled cells between 30 and 60 d (p = 0.77).
The phenotype of new cells was assessed using the immunohistochemical markers TuJ1 for immature and mature neurons and NeuN for mature neurons. Most of the BrdU-labeled cells were located in the GCL where ~75% expressed NeuN and ~80% expressed TuJ1 (Fig. 4). There was no effect of survival time or training condition on the percentage of BrdU-labeled cells that expressed either of these neuronal markers (p values > 0.05).
Corticosterone levels immediately after training were measured in separate groups of animals. Rats exposed to 800 trials of paired stimuli during trace conditioning emitted more CRs than those exposed to unpaired stimuli (F(1,16) = 14.0; p = 0.002), but their corticosterone levels were not different (paired, 184 ± 19 ng/ml; unpaired, 218 ± 19 ng/ml; p = 0.17). How-ever, concentrations in both trained groups were elevated compared with naive home-cage controls (84 ± 13; p values < 0.001).
Discussion
The data presented here demonstrate that learning to associate stimuli across time rather than training with discontinuous stimuli rescues adult-generated neurons from death. They also demonstrate that once rescued, the neurons persist for months in the dentate gyrus. As a group, animals exposed to only 200 trials of trace conditioning did not exhibit an increase in cell survival. However, there was a positive correlation between the performance of individual animals and cell survival (i.e., those animals that emitted more learned responses possessed more newly born cells). These data indicate that learning and not training is a sufficient condition for enhancing the lifespan of newly generated neurons and moreover that individual differences in associative learning predict whether new neurons will survive. In the second experiment, animals exposed to 800 trials of training on a trace eyeblink conditioning task exhibited significant learning, and the number of newly generated cells in the dentate gyrus after learning increased by ~40% when compared with the number of new cells in those exposed to explicitly unpaired stimuli. Double labeling with BrdU and the neuron-specific markers TuJ1 and NeuN revealed that ~80% of the new cells in the GCL of the dentate gyrus were neurons. The learning-induced increase in neuronal survival was long lasting because the cells that were labeled 1 week before learning remained in the hippocampus even 2 months after the animals had acquired the trace memory.
The number of adult-generated cells in the dentate gyrus of the hippocampus declines substantially within weeks of their generation, at least under standard laboratory conditions. This decrease is caused by death of newborn cells rather than dilution of BrdU (Cameron et al., 1993; Gould et al., 1999a; Dayer et al., 2003). However, there is evidence that neurons that survive beyond a few weeks persist at least 11 months in the rodent (Altman and Das, 1965; Kempermann et al., 2003), 12 weeks in the macaque (Gould et al., 2001), and 2 years in the human (Eriksson et al., 1998). Thus, the process of adult neurogenesis is not always a transient phenomenon and can lead to long-lasting changes in the number of new neurons in the adult hippocampus. The present findings are consistent with this notion because the number of BrdU-labeled cells decreased between 1 week and 1 month after labeling but not thereafter. Moreover, once rescued from death by learning, the new cells remain in the hippocampus for extended periods of time, perhaps permanently.
As discussed, the hippocampus is necessary for the formation of trace memories but becomes unnecessary for the maintenance of those memories within days of acquisition (Kim et al., 1995; Beylin et al., 2001; Takehara et al., 2003). The present data indicate that after learning the trace memory, new neurons persist for months, which is well beyond the time when the hippocampus is required for expression of the memory. Taken together, these data suggest that newly generated cells are affected by and may even participate in the initial acquisition of trace memories but that they assume another, as yet undetermined, role after learning has occurred. However, these data do not rule out the possibility that these cells continue to participate in the learning process. In fact, although the hippocampus is not necessary for the expression of trace memories beyond days of acquisition, it is often involved in the recall of remote memories for prolonged periods and perhaps for as long as some memories exist (Nadel and Moscovitch, 1997). Thus, newly generated neurons in the adult hippocampus may be a transient substrate by which trace memories are formed, but once incorporated into the existing circuitry, these cells could provide a means whereby previously learned information can be recalled more easily or related memories acquired more readily.
After training with only 200 trials of paired stimuli, animals that emitted more learned responses possessed more newly generated neurons. However, the mean number of cells remaining after 200 trials of paired stimuli was not different from that in response to 200 trials of unpaired stimuli and substantially less than after exposure to 800 trials of paired training. Thus, despite the correlation, it would appear that a shorter training episode is insufficient to rescue most cells from death, and many die in the days between training and perfusion. Also, we chose animals that reached a criterion of 60% CRs within 800 trials but did not use a criterion for animals exposed to only 200 trials. It may be important that we delivered all 200 trials within one session of training. Trial spacing is a critical factor in learning because acquisition is generally more efficient if trials are spaced apart rather than massed together (Kehoe and Gormezano, 1974; Rescorla, 1988). Trial spacing is particularly important during trace conditioning because the trace interval typically consists of the same context as that during the intertrial interval (Balsam, 1984; Shors, 2004). With short intervals and more trials, as used here, it is more difficult for the animal to distinguish between the CS and the US and the US and the next CS. Thus, it may be informative that those animals that were able to make this distinction early in training maintained more neurons in response to the learning experience. Perhaps 200 trials of trace conditioning would be sufficient to increase the number of new neurons if the trials were spaced such that animals more readily learned the association.
As a whole, these data indicate that new neurons are sensitive to the conditions of learning itself and are not responding indiscriminately to the training situation or the sensory stimulation associated with it. In this and previous studies, we showed that cell survival was not affected by exposure to explicitly unpaired stimuli, a training condition in which the two stimuli (CS and US) are presented one after the other but not in a temporally consistent manner (Gould et al., 1999a). Animals exposed to these training conditions do “learn”; they learn that the two stimuli do not occur together and are not temporally related to one another. However, they do not learn a positive relationship between the stimuli. One potential explanation remains, which is that exposure to unpaired stimuli is more stressful than learning the positive association. Because stress can reduce neurogenesis (Gould and Tanapat, 1999), it could be that the stressful nature of exposure to unpaired stimuli reduces cell survival. Several lines of evidence indicate that this is not a likely explanation. First, previous analysis found no difference in cell survival from animals exposed to unpaired stimuli versus those left in their home cage (Gould et al., 1999a). Second, here we found no difference in circulating levels of the stress hormone corticosterone at the time of training between animals exposed to paired versus unpaired training. Thus, exposure to unpaired training is not inherently more stressful than exposure to paired training, at least as measured with corticosterone. Overall, the data suggest that newly generated neurons in the dentate gyrus are not sensitive to all types of associative learning but rather are especially sensitive to stimuli that are presented in a temporally incongruous yet predictable manner.
Although an increasing number of studies suggest some relationship between adult neurogenesis and learning, the precise role of adult generated hippocampal neurons in learning is still debatable (Barnea and Nottebohm, 1996; Ambrogini et al., 2000; Dobrossy et al., 2003; Madsen et al., 2003; Raber et al., 2004; Shors, 2004). It has been proposed that newly generated neurons in the adult hippocampus must achieve full maturation before they become functional and influence behavior (Kempermann, 2002). However, it may be the relative immaturity of these cells that qualifies them for functions that more mature cells cannot accomplish (Gould et al., 1999b; Gould and Gross, 2002). Adult generated neurons have been shown to possess several structural and physiological characteristics in common with granule neurons generated during embryonic development. For example, they rapidly extend mossy fibers into area CA3 (Hastings and Gould, 1999) and exhibit robust long-term potentiation that, unlike mature granule cells, is insensitive to GABAergic inhibition (Wang et al., 2000; Snyder et al., 2001; Schmidt-Heber et al., 2004). These properties may be useful for processing and encoding stimulus events in real time.
Acknowledgments
This work was supported by National Institute of Mental Health Grants MH59970 and MH59740 and National Research Service Award fellowship MH63568.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in adult rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grade P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Balsam PD. Relative time in trace conditioning. Ann NY Acad Sci. 1984;423:211–227. doi: 10.1111/j.1749-6632.1984.tb23432.x. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc Natl Acad Sci USA. 1996;93:714–718. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood G, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontiguity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, LeMoal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gould E, Gross CG. Adult neurogenesis in mammals: some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999b;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Gormezano I. Effects of trial per session on conditioning of the rabbit’s nictitating membrane response. Bull Psychon Soc. 1974;2:434–436. [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely acquired, trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Markakis E, Gage F. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during early acquisition of trace conditioned responses. J Neurophysiol. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia, and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, Le Fevour A, Morhardt D, Curley J, Mizumatsu S, Vanden-Berg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells in the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesagaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on adult synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Solomon PR, VanderSchaaf ER, Weisz DJ, Thompson RF. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;104:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christis BR, Toni N, Palmer N, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogeneous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]