Abstract
Dopamine receptor signaling exhibits prominent plasticity that is important for the pathogenesis of both addictive and movement disorders. Psychoactive stimulants that activate the dopamine D1 receptor (drd1a) induce the rapid phosphorylation and activation of ERK1/2 in neurons of the nucleus accumbens and ventral striatum. This response is known to be dependent on the phosphatase inhibitor, DARPP-32 and appears critical for the sensitization of drd1a responses that contributes to addiction. Loss of dopamine input to the striatum, as in models of Parkinson's disease (PD), also results in a sensitization of responses to dopamine agonists that is manifest by increased activation of ERK1/2 in the dorsal striatum. Here we test if DARPP-32 is required for sensitization of drd1a responses in a PD model. In the normal dorsal striatum, there is minimal drd1a-mediated activation of ERK1/2, however, in the PD model there is robust drd1a-mediated activation of ERK1/2. In both wild-type and DARPP-32 knockout mice, drd1a robustly induces pERK1/2 throughout the dopamine-depleted striatum. These findings indicate that drd1a sensitization relevant for PD occurs by a novel mechanism that does not require DARPP-32.
Keywords: Amphetamine, Basal Ganglia, D1 [D-1], Dopamine, Dopamine receptor, Drug Abuse, Parkinson's disease, striatum
Introduction
Among the functions ascribed to the striatum is the use of context- and reward-related information to guide learned behavior (Schultz, 2002; Hikosaka et al., 2002). Glutamatergic cortical inputs to the striatum carry context-dependent information about ongoing behavior (Ding and Hikosaka 2006; Seger and Cincotta, 2006), and dopaminergic nigral inputs provide information about rewards (Schultz, 2002). Dopaminergic signals are thought to modulate corticostriatal synapses onto medium spiny projection neurons, and this plasticity is, in turn, thought to alter patterns of activity during learning (Schultz, 2002). Subversion of these processes is hypothesized to contribute importantly to addiction to psychostimulants (Nestler and Aghajanian, 1996; Greengard, 2001; Hyman et al., 2006), and movement disorders in Parkinson disease (PD) (Gerfen et al., 2002).
Activation of ERK1/2 is critical for long-term changes in synaptic plasticity at glutamatergic synapses (Thomas and Huganir, 2004), and has been implicated in both psychostimulant addiction (Valjent et al., 2000; 2005) and in dopamine receptor-agonist supersensitivity in PD models (Gerfen et al., 2002). Stimulation of corticostriatal glutamatergic inputs activate ERK1/2 in striatal neurons (Sgambato et al., ) through NMDA receptor coupling to Ca2+/calmodulin signaling systems, which activate the MAPkinasekinase (MEK) responsible for phosphorylation of ERK1/2. Psychostimulants have been suggested to amplify such NMDA-mediated activation of ERK1/2 by activating the drd1a dopamine receptor (Valjent et al, 2005). In this model, drd1a-receptors coupled to protein kinase A (PKA) regulate dopamine-receptor protein phosphatase inhibitor (DARPP-32, Greengard et al., 1999), whose inhibition of protein phosphatase 1, amplifies the activation of ERK1/2 (Valjent et al., 2005). This psychostimulant activation and associated behavioral effects are blocked by drd1a antagonists, by NMDA antagonists and by deletion of the genes encoding the drd1a receptor or DARPP-32 (Valjent et al., 2000, 2005). Together these data support the model that DARPP-32 functions to integrate coincident drd1a- and NMDA-receptor-mediated signaling to evoke plasticity.
While psychostimulants and drd1a agonists robustly activate ERK1/2 in neurons of the nucleus accumbens and ventral striatum, they are only weakly effective in the normal dorsal striatum (Valjent et al., 2000). However, after lesions of the dopamine projection in a model of PD, drd1a agonists produce a robust activation of ERK1/2 throughout the dorsal striatum (Gerfen et al., 2002). To assess whether this form of plasticity is also dependent on DARPP-32, we compared responses in wild type and DARPP-32 KO mice.
Methods
Animals and treatments
All animal procedures used in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Animals used in this study included mice with a genetic deletion (knockout) of the drd1a dopamine receptor (Drago et al., 1994) or the protein phosphatase inhibitor, DARPP-32 (Fienberg et al., 1998). Both mice are on a C57/BL6 background and maintained with either homozygous or heterozygous breeding pairs. Wild-type control animals were age-matched from the breeding colonies used for the knockout mice. For these studies, both male and females were used. The genotype of each animal was determined from tail clips using PCR with appropriate primer sequences.
Two different experimental paradigms were used, one in which psychostimulant drugs were administered to the animals, the other in which drd1a agonists were administered to animals with unilateral lesions of the nigrostriatal dopamine system. To produce these lesions, mice of approximately 25−35 g in weight were anaesthetized with Nembutal, placed in a stereotaxic frame and a unilateral lesion was produced by infusing 6-OHDA (8μg in 1μl) into the substantia nigra. Animals were returned to their home cages, food supplemented with grapes for 2−3 weeks, during which the mice had unrestricted access to food and water.
Drug treatments used in these studies involved intraperitoneal (i.p.) administration of one of the following drugs: vehicle (0.9% saline), the psychostimulants d-amphetamine (10 mg/kg) or cocaine (20 or 30 mg/kg), the drd1a agonist (±) 6-Chloro-PB (6-chloro-7,8-dihyroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-bezazepine-hydrobromide: SKF-81297, item #S-143, Sigma Chemical Company, St. Louis MO), or L-DOPA (20 mg/kg, L,3,4-Dihydroxyphenylanine methyl ester hydrochloride, item #D1507, Sigma Chemical Company) with benserazide hydrochloride (12 mg/kg, item# 7283 Sigma Chemical Company).
Fifteen minutes following drug treatment, the animals were asphyxiated with carbon dioxide and perfused transcardially with 4% formaldehyde in phosphate buffer, pH 7.4. Their brains were removed and processed for immunohistochemistry.
Histology
Following perfusion, the brains were post-fixed overnight at 4°C by immersion in the formaldehyde solution described above. The brains were then transferred to a 20% sucrose solution (in PBS, pH 7.4) and were kept at 4°C until they sank. Brains were frozen and sectioned on a sliding microtome, and a series of 40μm coronal sections from the frontal pole through the midbrain were collected and stored at 4°C in PBS until processed for immunohistochemistry. Selected free-floating sections were incubated overnight in blocking solution (0.2% Triton X-100, 2% normal serum in PBS) containing primary antisera for TH (1:250, Pel-Freez Biologicals, Rogers, AK), DARPP-32 (1:4000, Chemicon International, Temecula, CA) or phospho-ERK1/2 (1:250, Cell Signaling Technology). The primary antibodies were visualized using the Vectastain ABC elite protocol (Vector laboratories) and 3,3’-diaminobenzidine.
Microscopic digital imaging was used to analyze immunoreactivity in processed brain sections. When post-imaging processing was performed, images taken from different animals were processed together to alter brightness, contrast and color.
Western Blots
Whole frozen brains stored at −70°C were briefly warmed to −20°C before striatal tissue samples were collected. Protein extracts were purified by sonication in 10x v/w 20mM Tris-HCl pH7.5 containing 2mM EDTA, 20mM glycerophosphate, 1mM Na3VO4, 2mM NaF and 1x Complete protease inhibitor mix (11 873 580 001, Roche, Germany) then centrifuged at 15,000xg for 30 min at 4°C. Supernatants were stored at −70°C. Protein concentrations were determined in triplicates using a bicinchoninic acid (BCA) assay kit (Pierce, Rockford IL). Samples of 18μg protein were separated by SDS-PAGE using 4−12% gels and blotted onto 0.45μm pore size PVDF using the NuPAge Novex (Invitrogen, Carlsbad, CA) electrophoresis system following the supplied protocols. Immunohistochemistry was performed using the phospho-Erk1/2 1:1000 (#9101, Cell Signaling Tech., Danvers, MA), DARPP-32 1:3000 (AB1656, Millipore, Billerica, MA) or tyrosine hydroxylase 1:4000 (#P40101 Pel-Freez, Rogers, AR (Sigma, St Louis, MO) primary antibodies in 20mM Tris HCl pH7.5 containing 0.9% w/v NaCl , 0.1% v/v Tween-20 and 5% w/v non-fat dry milk. Antibody binding was detected using anti-rabbit-IgG-HRP secondary antibody 1:20,000 (#7074 Cell Signaling Technology, Beverly MA) and visualized by chemiluminescence (Supersignal, Pierce) and autoradiographic film (Kodak, Rochester NY). Films were digitized and analyzed using NIH-Image to determine the size of the band using the area under the plot profile for each band. If bolts were re-probed they were first striped using Restore (Pierce) at 37°C for 30 min, then checked by incubating with secondary antibody at 1:10,000 for 1 hr and visualized using chemiluminescence.
Results
Requirement for drd1a receptors and DARPP-32 for psychostimulant activation of ERK1/2
Wild-type mice and mice with genetic deletions of the drd1a dopamine receptor or DARPP-32 were treated with the psychostimulants d-amphetamine (10 mg/kg, i.p.) or cocaine (20 mg/kg, i.p.) and perfused 15 minutes later. For each treatment group 8 animals were used. Subsequently, the striatum was analyzed for neurons immunoreactive for phosphorylated ERK1/2 (phospho-ERK1/2). In wild-type mice, d-amphetamine treatment resulted in numerous neurons immunoreactive for phosphorylated ERK1/2, which were located in the nucleus accumbens (Figure 1). In addition, there were some immunoreactive neurons in the medial region of the dorsal striatum. These neurons were most abundant adjacent to the ventricle and their density decreased rapidly such that in the lateral region only a few scattered neurons were observed (Figure 1). Knockout of either drd1a or DARPP-32 resulted in a reduction of both the numbers and intensity of labeling of phospho-ERK1/2 in the nucleus accumbens compared to treated wild-type mice (Figure 1). In both drd1a and DARPP-32 knockout mice, phospho-ERK1/2 immunoreactive neurons were present in the medial dorsal striatum. As in wild-type mice, in both of the knockout mice there were very few phospho-ERK1/2 labeled neurons in the lateral dorsal striatum. Counts of amphetamine-induced phospho-ERK1/2 immunoreactive neurons in the different regions of the striatum show no significant difference in the lateral or medial dorsal striatum between wild type or Drd1a or DARPP-KO animals, whereas in the nucleus accumbens there is a 66% reduction in Drd1 KO and 61% percent reduction in DARPP KO mice compared to wild type (Table 1).
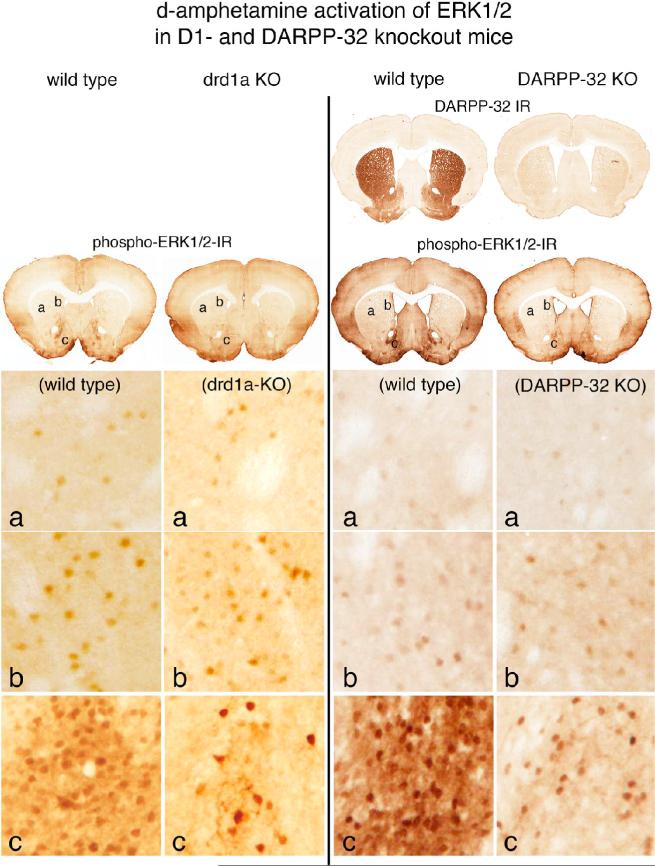
Figure 1.
Psychostimulant-induced activation of ERK1/2 in drd1a- and DARPP-32-knockout mice. Effects of d-amphetamine treatment (10mg/kg) are compared between wild type and drd1a knockout (KO) mice and between wild type and DARPP-32 KO mice, in which DARPP-32 immunoreactivity (IR) in the striatum is absent. Activation of ERK1/2 is indicated in coronal brain sections by neurons displaying immunoreactive phosphorylated ERK1/2 (phospho-ERK1/2-IR). In the dorso-lateral striatum (a) and in the dorso-medial striatum (b) the numbers of scattered neurons with d-amphetamine-induced immunoreactive phospho-ERK1/2 is similar in the wild type and drd1a-KO and DARPP-32 KO animals. In the shell of the nucleus accumbens (c), there are numerous d-amphetamine-induced phospho-ERK1/2 immunoreactive neurons in the wild type mice, whereas in both the drd1a-KO and DARPP-32-KO mice the numbers of such neurons is markedly reduced. These data indicate that psychostimulant activation of ERK1/2 mediated by drd1a and DARPP-32 is restricted to the nucleus accumbens.
Table 1.
d-amphetamine activation of ERK1/2 in striatal neurons in wild type, Drd1a- and DARPP-32 KO mice.
| STRIATAL REGION | Wild type | drd1a-KO | p value ** <0.01 | Wild type | DARPP32-KO | p value ** <0.01<n.s. |
|---|---|---|---|---|---|---|
| dorsal lateral | 5.2 | 6.0 | 0.268 | 4.7 | 5.5 | n.s.0.268 |
| dorsal medial | 19.7 | 21.3 | 0.322 | 18.7 | 21.0 | n.s.0.294 |
| accumbens | 55.8 | 19.3 | **0.0011 | 47.3 | 18.5 | **0.0011 |
Counts of neurons showing phospho-ERK1/2 in different regions of the striatum and nucleus accumbens from animals in treatment groups described in this study. Treatment groups with d-amphetamine (10 mg/kg, ip) included wild type and drd1a- and DARPP-32-knockout animals. Cell counts are the average from 6 animals per treatment group (n=6) measured in a 100 um2 area in each region. Wilcoxon ranks sums test show that there is only a significant reduction (** p value <0.01) in amphetamine-induction of phospho-ERK1/2 neurons in the nucleus accumbens in both drd1a-KO and DARPP-32 KO animals compared to wild type.
Cocaine (20- or 30 mg/kg) treatment produced phospho-ERK1/2 responses similar to those produced by d-amphetamine (10 mg/kg) in terms of the distribution of phospho-ERK1/2 immunoreactive neurons, although the level of immunoreactivity was qualitatively lower on a per cell basis with cocaine treatment (supplementary Figure 1).
Transgenic mice in which EGFP is expressed under the control of the promoter for either Drd1a- or Drd2-genes with Bacterial Artificial Chromosome (BAC)-constructs (Gong et al., 2003) were used to determine which striatal neuron types displayed phospho-ERK1/2 immunoreactivity following d-amphetamine treatment (10 mg/kg). Double labeling using a red fluorochrome to visualize phospho-ERK1/2 immunoreactivity and a green fluorochrome to visualize EGFP immunoreactivity was used to determine co-localization of the markers. The relative numbers of amphetamine-induced phospho-ERK1/2 immunoreactive neurons in different striatal regions was similar to that described above. In the Drd1a-BAC-EGFP mice, greater than 90% of striatal neurons displaying phospho-ERK1/2 co-localized with neurons expressing EGFP immunoreactivity, in all regions of the striatum (figure 2 and Table 2). On the other hand, in the Drd2-BAC-EGFP mice, fewer than 10% of neurons displaying phospho-ERK1/2 immunoreactivity co-expressed EGFP (Figure 3 and Table 2).
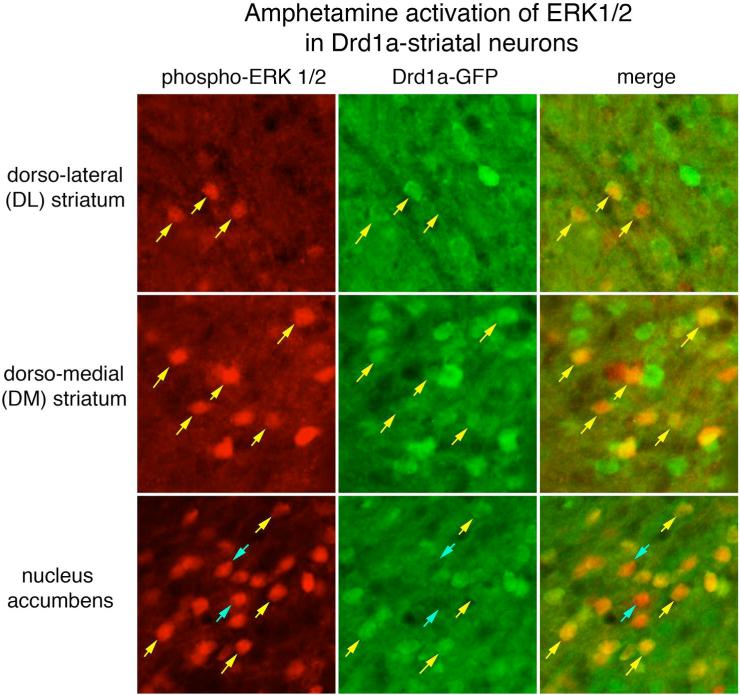
Figure 2.
d-amphetamine activation of ERK1/2 in Drd1a-expressing striatal neurons. Drd1a-BAC-EGFP transgenic mice were treated with d-amphetamine (10 mg/kg) and brain sections containing the striatum were processed for co-localization of phospho-ERK1/2-IR (red fluorochrome) and GFP-IR (green fluorochrome). A 100 um2 area of the dorso-lateral (DL), dorso-medial (DM) and nucleus accumbens is shown. The numbers of phospho-ERK1/2 immunoreactive neurons varies from few in the DL to many in the nucleus accumbens. In each region nearly all phospho-ERK1/2 IR neurons co-localize with GFP (yellow arrows), which is produced in Drd1a striatal neurons. There are some rare phospho-ERK1/2 IR neurons that are GFP-IR negative ( blue arrows ) clearly evident as dark lacunae in the back ground of GFP fluorescence. These data indicate nearly all striatal neurons in which ERK1/2 is activated following d-amphetamine treatment express the Drd1a receptor ( see cell counts in Table 2).
Table 2.
d-amphetamine activation of ERK1/2 in Drd1a-and Drd2-expressing striatal neurons.
| STRIATAL REGION | pERK-IR/Drd1a-GFP + | pERK-IR/Drd1a-GFP − | %Drd1a-GFP + | pERK-IR/Drd1a-GFP + | pERK-IR/Drd1a-GFP − | %Drd2-GFP + |
|---|---|---|---|---|---|---|
| dorsal lateral | 4.5 | 0.25 | 95% | 0.25 | 7.25 | 3.3% |
| dorsal medial | 12.5 | 1 | 93% | 1.0 | 15.75 | 6.0% |
| accumbens | 35.2 | 3.25 | 92% | 7 | 63.75 | 9.6% |
Counts phospho-ERK1/2-immunreaactive (pERK-IR) striatal neurons in different regions following d-amphetamine (10 mg/kg) in Drd1a-GFP and Drd2-GFP transgenic mice. Cell counts are the average numbers of pERK1/2-IR cells that co-localize with GFP-IR in a 100 um2 area in each region. For each region the percent of pERK-IR cells is calculated by summing pERK-IR positive cells, which are either GFP positive (GFP+) or GFP negative (GFP-). While, the average numbers of pERK-IR cells varies from few in the dorsolateral striatum to many in the nucleus accumbens, more than 90% co-localize with GFP in Drd1a-GFP mice whereas less than 10% co-localize with GFP in Drd2-GFP mice. These data indicate that over 90% of striatal neurons in which ERK1/2 is activated following d-amphetamine treatment express the Drd1a receptor.
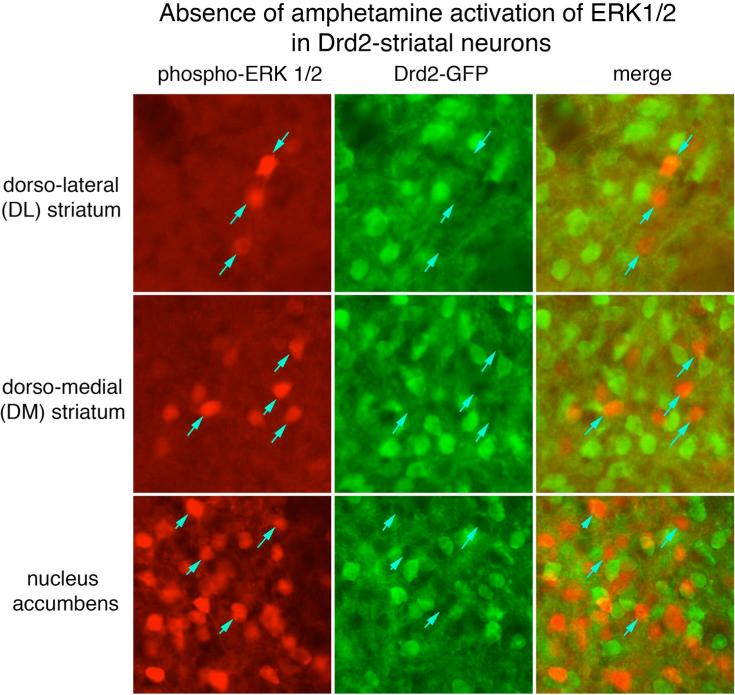
Figure 3.
d-amphetamine activates ERK1/2 in few Drd2-expressing striatal neurons. Drd2-BAC-EGFP transgenic mice were treated with d-amphetamine (10 mg/kg) and brain sections containing the striatum were processed for co-localization of phospho-ERK1/2-IR (red fluorochrome) and GFP-IR (green fluorochrome). A 100 um2 area of the dorso-lateral (DL), dorso-medial (DM) and nucleus accumbens is shown. The numbers of phospho-ERK1/2 immunoreactive neurons varies from few in the DL to many in the nucleus accumbens. In each region nearly all phospho-ERK1/2 IR neurons do not express GFP, which is produced in Drd2-striatal neurons. These data indicate that very few striatal neurons in which ERK1/2 is activated following d-amphetamine treatment express the Drd2 receptor ( see cell counts in Table 2).
Drd1a agonist activation of ERK1/2 in the intact and dopamine-depleted striatum
Wild-type and DARPP-32 knockout mice underwent unilateral 6-OHDA lesions of the nigrostriatal dopamine system. 3 wks after the lesion, they were treated with the full D1 receptor agonist, SKF 81297 (5 mg/kg) and perfused 15 minutes later. For each treatment group 8−10 animals were used. The striatum was then analyzed for neurons immunoreactive for phosphorylated ERK1/2. Unilateral degeneration of the nigrostriatal dopamine pathway was confirmed by the absence of tyrosine hydroxylase immunoreactive (TH-IR) neurons in the substantia nigra and in axon terminals in the striatum (Figure 4). In the dopamine-intact striatum (contralateral to the lesion), phospho-ERK1/2 immunoreactive neurons were evident in the nucleus accumbens, but only scattered neurons were present in the dorsal striatum. This pattern of labeling resembles that seen with psychostimulant treatments in the dopamine-intact striatum in the absence of 6-OHDA lesions, as described above. In the dopamine-depleted striatum, phospho-ERK1/2 immunoreactive neurons were abundant and spread throughout both the dorsal striatum and the nucleus accumbens. This pattern of drd1a-agonist-induced activation of ERK1/2 was identical in wild-type and DARPP-32 KO mice.
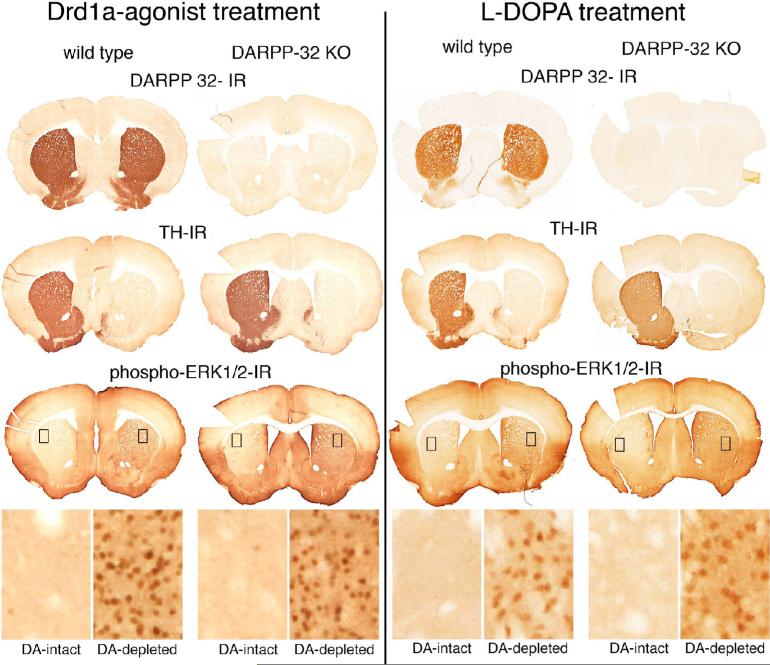
Figure 4.
Drd1a-agonist or L-DOPA-activation of ERK1/2 in the dopamine (DA) depleted striatum does not involve DARPP-32. Comparison of coronal brain sections at the level of the rostral striatum from wild type and DARPP-32 knockout (KO) mice, with unilateral lesions of the nigrostriatal dopamine system and treated with a drd1a-agonist (SKF81298, 5 mg/kg, 1 day) or L-DOPA (20 mg/kg with 12 mg/kg benserazide, 10 days). DARPP-32 immunoreactivity (IR) labels neurons in the striatum in wild type mice, which are unlabeled in DARPP-32 KO mice. Unilateral lesion of the nigrostriatal dopamine pathway in these animals is shown by the absence of tyrosine hydroxylase (TH)-IR the axonal terminals in the right striatum. Activation of ERK1/2 in response to either drd1a agonist treatment (Left side figures) or L-DOPA treatment (right side figures), is demonstrated by phospho-ERK1/2-IR, throughout the dopamine depleted striatum. High power images from the dorsolateral striatum (inset boxes, 100um wide) show few to no phospho-ERK1/2 IR neurons in the DA-intact striatum. In contrast there are numerous phospho-ERK1/2 IR neurons in the DA-depleted striatum in both the wild type and DARPP-32 KO animal. Cell counts are provided in Table 2.
In a recent paper Santini et al. (2007) have implicated DARPP-32 activation of ERK1/2 as critical to the induction of L-DOPA-induced dyskinesia (LIDs). To determine whether there was a difference between treatment with the drd1a-agonist and L-DOPA wild-type and DARPP-32 knockout mice with unilateral 6-OHDA lesions of the nigrostriatal dopamine system were treated with L-DOPA (20 mg/kg L-DOPA with 12 mg/kg benserazide) for 1 or 10 days. Results with L-DOPA treatment were identical to those with the drd1a-agonist treatment. In both wild-type and DARPP-32 knockout animals there was robust labeling of phospho-ERK1/2 immunoreactive neurons throughout the dopamine-depleted striatum (Figure 4). Cell counts showed no significant difference between the numbers of phospho-ERK immunoreactive neurons in the dopamine-depleted striatum between wild type and DARPP-32 knockout mice treated with either the drd1a-agonist or L-DOPA (Table 3 and Table 4).
Table 3.
| Wild type | Wild type | DARPP-32 KO | DARPP-32 KO | Comparison of WT and KO DA-depleted striatum | |
|---|---|---|---|---|---|
| STRIATAL REGION | DA-intact | DA-depleted | DA-intact | DA-depleted | p value ** <0.01<n.s. |
| dorsal lateral | 3.5 | 46.5 | 4.2 | 50.5 | n.s.0.220 |
| dorsal medial | 13.5 | 49.2 | 10.0 | 56.2 | n.s.0.078 |
| accumbens | 24.7 | 50.8 | 20.3 | 49.2 | n.s.0.409 |
Treatment groups receiving drd1a-agonist (SKF81298, 5 mg/kg) had unilateral 6-OHDA lesions of the nigrostriatal dopamine pathway, phospho-ERK1/2 immunoreactive neurons were counted in the DA-intact and DA-depleted striatum in wild type and DARPP-32 knockout animals. Cell counts are the average from 6 animals per treatment group (n=5) measured in a 100 um2 area in each region. Wilcoxon ranks sums test were performed to compare the DA-depleted striatum from the wild type and DARPP-32 KO animals. There was no significant difference (n.s., p value > 0.01) between wild type and DARPP-32 KO animals in the numbers of neurons displaying drd1a-agonist activated ERK1/2 in the DA-depleted striatum.
Table 4.
| Wild type | Wild type | DARPP-32 KO | DARPP-32 KO | Comparison of WT and KO DA-depleted striatum | |
|---|---|---|---|---|---|
| STRIATAL REGION | DA-intact | DA-depleted | DA-intact | DA-depleted | p value ** <0.01<n.s. |
| dorsal lateral | 6.6 | 51.4 | 3 | 58.2 | n.s.0.183 |
| dorsal medial | 16.8 | 43.8 | 7.4 | 50.8 | n.s.0.111 |
| accumbens | 27.8 | 39.6 | 20.0 | 46.0 | n.s.0.21 |
Treatment groups receiving L-DOPA (20 mg/kg plus 12 mg/kg benseraizde for 10 days) had unilateral 6-OHDA lesions of the nigrostriatal dopamine pathway, phospho-ERK1/2 immunoreactive neurons were counted in the DA-intact and DA-depleted striatum in wild type and DARPP-32 knockout animals. Cell counts are the average from 5 animals per treatment group (n=5) measured in a 100 um2 area in each region. Wilcoxon ranks sums test were performed to compare the DA-depleted striatum from the wild type and DARPP-32 KO animals. There was no significant difference (n.s., p value > 0.01) between wild type and DARPP-32 KO animals in the numbers of neurons displaying L-DOPA activated ERK1/2 in the DA-depleted striatum.
The study by Santini et al. (2007) used Western immuno-blot analysis to determine the relative L-DOPA-induced activation of ERK1/2. We analyzed wild type (N=3) and DARPP-32 knockout (N=3) mice with unilateral 6-ODHA lesions of the nigrostriatal dopamine pathway treated for 10 days with L-DOPA (20 mg/kg L-DOPA with 12 mg/kg benserazide) by Western immuno-blot. This analysis showed a robust actvitation of pERK2 in the DA-lesioned striatum in both wildtype and DARPP-32 knockout animals (Figure 5). This immunoblot data is consistent with the immunohistochemical data described above.
Figure 5.
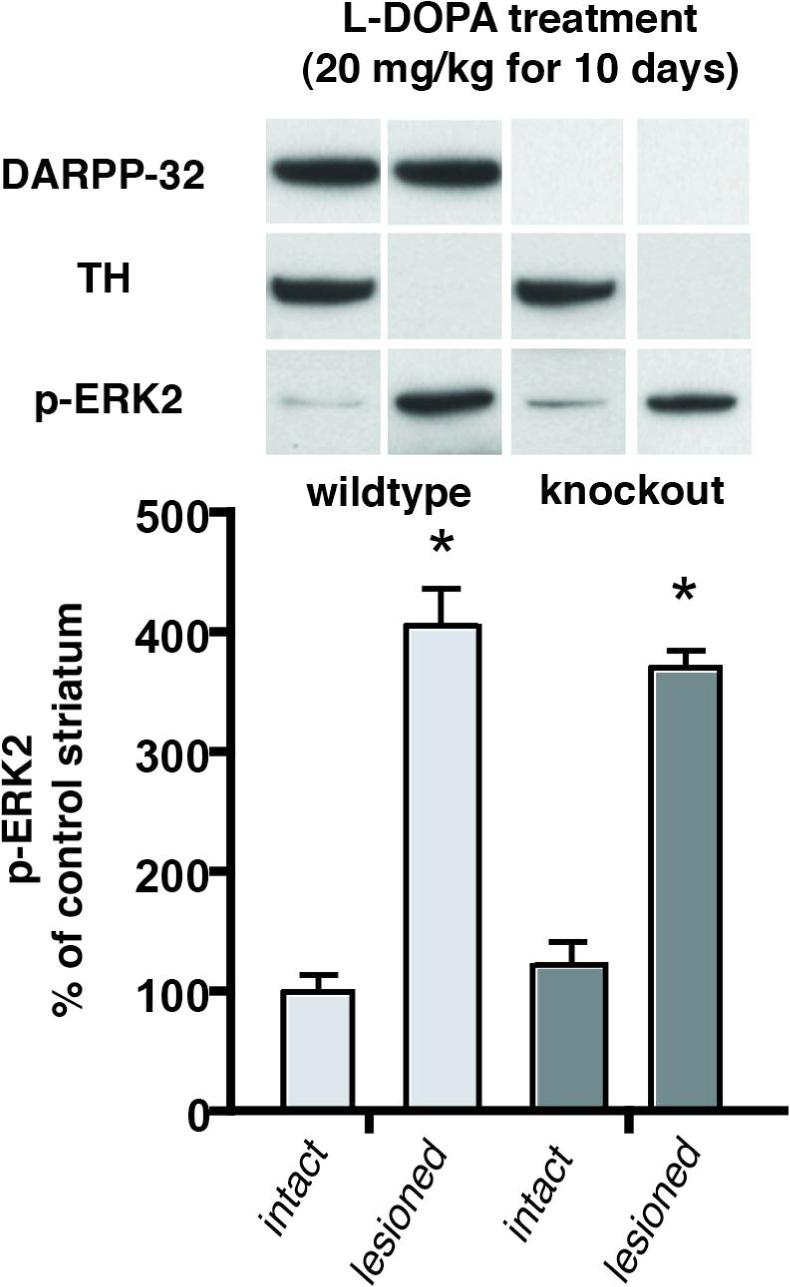
L-DOPA-activation of ERK2 in the dopamine depleted striatum is not significantly reduced in DARPP-32 knockout mice. Western immunoblot data from wildtype (n=7) and DARPP-32 knockout (n=5) animals with unilateral 6-OHDA lesions of the nigrostriatal dopamine pathway treated for 10 days with L-DOPA (20 mg/kg with 12 mg/kg benserazide). Animals were killed 30 min after the last injection and the dissected striatum were processed by Western blotting to determine levels of immunoreactivity for DARPP-32, tyrosine hydroxylase (TH) and phosphorylated ERK2 (pERK2). There is robust activation of pERK2 in the dopamine-depleted compared to the intact striatum of both wild type and DARPP32-KO animals (* p<0.05). On the other hand, there is no significant difference in the activation of pERK2 in the dopamine-lesioned striatum comparing wildtype (WT) and DARPP-32 knockout (KO) animals (percent above intact control: WT: 406% KO: 371%, p>0.05)
To further examine the role of the drd1a-dopamine receptor in activation of ERK1/2, drd1a knockout mice were treated with unilateral lesions of the nigrostriatal dopamine system were treated with either the drd1a-agonist ( SKF 81297, 5 mg/kg) or with L-DOPA ( 20 mg/kg with 12 mg/kg benserazide). Treatment with the drd1a-agonist produced no labeling of phoshpo-ERK1/2 immunoreactive neurons in either the dopamine-intact or dopamine depleted striatum (supplemental Figure 2) . This confirms that drd1a-agonist activation of ERK1/2 in the dopamine-depleted striatum is dependent on the drd1a-dopamine receptor. Treatment with L-DOPA resulted in no phospho-ERK1/2 immunoreactive neurons in the dopamine-intact striatum and labeling of only scattered large neurons in the dopamine-depleted striatum (supplementary Figure 2). The phospho-ERK1/2 immunoreactive neurons co-localized choline acetyl transferase (ChAT) immunoreactivity (data not shown), indicating that these neurons are striatal cholinergic interneurons. These neurons express both the D2- and D5-dopamine receptor, which is likely responsible for their activation by L-DOPA treatment, in the Drd1a-KO mice.
Discussion
The present study indicates that DARPP-32 is not required for a specific form of dopamine supersensitivity that is relevant for Parkinson's Disease. Signaling events that couple dopamine drd1a-activity to increases in phosphorylated ERK1/2 in neurons of the dorsal striatum undergo striking adaptations in response to loss of dopamine projections from the substantia nigra. In the normal dorsal striatum, selective D1 agonists or cocaine result in modest increases in pERK1/2 that are restricted to a small subset of neurons. After denervation, D1 agonists evoke marked increases of pERK1/2 in a large population of neurons in the dorsal striatum. While DARPP-32 is required for D1-agonist induced increases of pERK1/2 in the ventral striatum and nucleus accumbens, the marked increase in pERK1/2 in the dorsal striatum consequent to loss of dopamine projections is identical in wt and DARPP-32 KO mice. This finding indicates that the adaptations that underlie denervation-induced drd1a supersensitivity in the dorsal striatum do not require DARPP-32.
Several lines of evidence support the notion that drd1a signaling that activates pERK1/2 is fundamentally different in the major populations of drd1a neurons in the ventral versus the dorsal striatum. In both drd1a- and DARPP-32 knockout mice, the acute effects of cocaine and amphetamine to induce pERK1/2 are significantly reduced in the nucleus accumbens [(this study and (Valjent et al., 2005)]. However, psychostimulant effects on induction of pERK1/2 are very modest in the dorsal striatum and this pERK1/2 response persists in drd1a- and DARPP-32 knockout animals. Moreover, even in the ventral striatum, there is a small population of neurons that show drd1a-induced pERK1/2 in drd1a or DARPP-32 KO mice. Accordingly, psychostimulant activation of ERK1/2 in the dorsolateral and dorsomedial striatum, as well as some of the activation in the nucleus accumbens, appears not to require drd1a receptors or DARPP-32. As with psychostimulants, either selective drd1a-agonist treatment or direct stimulation of the nigrostriatal dopamine pathway induces pERK1/2 in the nucleus accumbens, but does not produce activation in the dorsal striatum (Gerfen et al., 2002). In these same studies, either drd1a agonists or stimulation of the nigrostriatal dopamine system resulted in robust induction of immediate early genes (IEGs) in drd1a neurons throughout the ventral and dorsal striatum, confirming that neurons of the dorsal striatum are being stimulated.
Drd1a signaling in the denervated dorsal striatum appears to be mechanistically most similar to that seen in the small population of drd1a neurons of the normal dorsal striatum that show drd1a-induced increases in pERK1/2. This is inferred from the observations that drd1a-pERK1/2 signaling in both populations of neurons is independent of DARPP-32. Importantly, this observation excludes the alternative possibility that the mechanism that transduces drd1a signals to pERK1/2 in the lesioned dorsal striatum is the same as that used in the ventral striatum of normal animals. The molecular pathways that mediate this DARPP-32 independent response in both the intact and denervated dorsal striatum remain to be identified.
Activation of ERK1/2 has been implicated in plasticity in a variety of brain regions, including the cerebral cortex, amygdala, spinal cord and nucleus accumbens, and contributes to several forms of learning and memory (Thomas and Huganir, 2004). What is common in these diverse regions is the dependence of activation of ERK1/2 on NMDA glutamatergic excitatory synaptic inputs. The normal function of dopamine in this process is to integrate convergent excitatory inputs, by regulating activation of ERK1/2 through a DARPP-32-mediated mechanism. Several features of drd1a-mediated activation of ERK1/2 in the dopamine-depleted striatum are unique including its independence of NMDA receptor function (Gerfen et al., 2002) and independent of DARPP-32 (this study). The absence of NMDA and DARPP-32 regulatory controls underscores the unique features of signaling in dorsal striatal neurons, and anticipates novel forms of synaptic plasticity.
Adaptations of drd1a signaling are likely to contribute to the pathogenesis of PD. Neurons that express drd1a in the dorsal striatum are part of the “direct pathway” that projects to the substantia nigra pars reticula, and are important for voluntary motor responses. Loss of dopamine input alters the function of direct-pathway neurons, and this is linked to changes in motor behavior observed in PD. Since these neurons normally function to disinhibit (activate) motor circuits, their enhanced drd1a-pERK1/2 signaling may represent a homeostatic adaptation to increase this drive, and as such may be linked to increased locomotor activity in response to D1-agonists. We have previously proposed that these adaptations contribute to altered motor behaviors, including dyskinesias or “on-off syndrome”, in PD patients who have been chronically treated with dopamimetic agonists (Gerfen et al., 2002). Consistent with this proposal two recent studies (Santini et al., 2007; Westin et al., 2007) have demonstrated the involvement of activation of ERK1/2 in the development of L-DOPA induced dyskinesia in rodent models of PD. The Santini et al. (2007) study also proposed that DARPP-32 is critical in mediating L-DOPA-activation of ERK1/2 as there is a significant reduction in ERK1/2 activation in DARPP-32 KO mice compared to wild type as determined with western blot analysis. In the current study, using the same L-DOPA treatment paradigm, we report that there is no significant difference between wild type and DARPP-32 KO mice in the numbers of striatal neurons displaying phospho-ERK1/2 immunoreactivity nor in the level of phospho-ERK2 analyzed with Western blots in the dopamine-depleted striatum. Given our finding of robust L-DOPA induced activation of ERK1/2 in the majority of Drd1a-striatal neurons in DARPP-32 KO mice, we conclude that DARPP-32 is not critical to such activation. On the other hand, given the demonstrated critical role that DARPP-32 has in mediating a wide variety of signal transduction mechanisms, it is possible that DARPP-32 may be involved in mechanisms underlying altered synaptic plasticity responsible for L-DOPA induced dyskinesias.
In summary, two major conclusions may be drawn from the present study. First, the mechanism involving DARPP-32 regulation of Drd1a-mediated activation of ERK1/2 is regionally restricted within the striatum to the nucleus accumbens, a part of the ventral striatum. Second, the drd1a-mediated activation of ERK1/2 in the dopamine-depleted dorsal striatum does not involve an “amplification” of the DARPP-32-mediated regulation of Drd1 signaling that occurs in the nucleus accumbens. Rather, it represents a novel signaling pathway that is most similar to drd1a signaling seen in a small population of dorsal striatal neurons in intact animals that it is independent of DARPP-32. Together, these results demonstrate the existence of regionally distinct cellular and molecular mechanisms within the striatum that mediate drd1-signaling.
Acknowledgements
DARPP-32 KO mice were generously provided by Professor Paul Greengard, Rockefeller University. We would like to acknowledge the excellent technical support of Ron Harbaugh, Michelle Tenace and Alex Cummins. We also thank Steve Wise for providing statistical analysis of the cell count data. Supported by the NIMH Intramural Research Program (CRG) and by DA11742 ((PW).
Supplementary Material
Supplementary Figure 1. Comparison of d-amphetamine and cocaine activation of ERK1/2 in wild type and DARPP-32-knockout mice. Effects of two psychostimulants, d-amphetamine (10mg/kg) and cocaine ( 20 mg/kg) on activation of ERK1/2 are compared between wild DARPP-32 KO mice. Activation of ERK1/2 is indicated in coronal brain sections by neurons displaying immunoreactive phosphorylated ERK1/2 (phospho-ERK1/2-IR). The regional pattern of ERK1/2 activation is similar for both psychostimulants, although the per cell labelling appears to be more robust with damphtamine treatment. Higher magnification images ( the highest magnification shows an area 100um2) are shown for the nucleus accumbens. In the DARPP-32 KO mice there is a significant reduction of the numbers of phospho-ERK1/2 IR neurons in the nucleus accumbens for both psychostimulant treatments.
Supplementary Figure 2. Effects of Drd1a-agonist and L-DOPA in Drd1a-KO mice Drd1a knockout mice with unilateral lesions of the nigrostriatal dopamine system were treated with either the drd1a-agonist ( SKF 81297, 5 mg/kg) or with L-DOPA ( 20 mg/kg with 12 mg/kg benserazide). Lesion of the right dopamine input to the right striatum is evident by the absence of TH-IR. Treatment with the drd1a-agonist produced no labeling of phoshpo-ERK1/2 immunoreactive neurons in either the dopamine-intact or dopamine depleted striatum Treatment with L-DOPA resulted in no phospho-ERK1/2 immunoreactive neurons in the dopamine-intact striatum and labeling of only scattered large neurons in the dopamine-depleted striatum.
Supplementary Figure 3. Images of Western immunoblot used for data presented in Figure 5. Protein samples (18ug each) from theintact- and lesioned-striatum of wildtype (N=3) and DARPP-32 KO (N=3) animals were loaded onto one gel, separated, blotted and probed for phospho-ERK1/2 immunoreactivity. The blot was then stripped and re-probed for DARPP32- and tyrosine hydroxylase (TH)-immunoreactivity. Digitized images of the bands were used for quantitative analysis. The control value (100%) for each protein was the average value of the bands for the wild-type intact striatum. For illustrative purposes one band for each condition from these images were used in Figure 5.
References
- Brown P, Gerfen CR. Plasticity within striatal direct pathway neurons following neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK1/2/MAP Kinase pathway. J Comp Neurol. 2006;498:415–430. doi: 10.1002/cne.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. J Neurosci. 2006;26:6695–703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowitz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, Bartlett PF, Jose PA, Sibley DR, Westphal H. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc. Natl. Acad. Sci. (USA) 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–42. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 Dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP Kinase. J Neuroscience. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–30. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–22. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cereb Cortex. 2006;16:1546–55. doi: 10.1093/cercor/bhj092. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5(3):173–83. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–9. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–6. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal Pattern of Striatal ERK1/2 Phosphorylation in a Rat Model of L-DOPA-Induced Dyskinesia and the Role of Dopamine D1 Receptors. Biol Psychiatry. 2007 Jul 25; doi: 10.1016/j.biopsych.2006.11.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Comparison of d-amphetamine and cocaine activation of ERK1/2 in wild type and DARPP-32-knockout mice. Effects of two psychostimulants, d-amphetamine (10mg/kg) and cocaine ( 20 mg/kg) on activation of ERK1/2 are compared between wild DARPP-32 KO mice. Activation of ERK1/2 is indicated in coronal brain sections by neurons displaying immunoreactive phosphorylated ERK1/2 (phospho-ERK1/2-IR). The regional pattern of ERK1/2 activation is similar for both psychostimulants, although the per cell labelling appears to be more robust with damphtamine treatment. Higher magnification images ( the highest magnification shows an area 100um2) are shown for the nucleus accumbens. In the DARPP-32 KO mice there is a significant reduction of the numbers of phospho-ERK1/2 IR neurons in the nucleus accumbens for both psychostimulant treatments.
Supplementary Figure 2. Effects of Drd1a-agonist and L-DOPA in Drd1a-KO mice Drd1a knockout mice with unilateral lesions of the nigrostriatal dopamine system were treated with either the drd1a-agonist ( SKF 81297, 5 mg/kg) or with L-DOPA ( 20 mg/kg with 12 mg/kg benserazide). Lesion of the right dopamine input to the right striatum is evident by the absence of TH-IR. Treatment with the drd1a-agonist produced no labeling of phoshpo-ERK1/2 immunoreactive neurons in either the dopamine-intact or dopamine depleted striatum Treatment with L-DOPA resulted in no phospho-ERK1/2 immunoreactive neurons in the dopamine-intact striatum and labeling of only scattered large neurons in the dopamine-depleted striatum.
Supplementary Figure 3. Images of Western immunoblot used for data presented in Figure 5. Protein samples (18ug each) from theintact- and lesioned-striatum of wildtype (N=3) and DARPP-32 KO (N=3) animals were loaded onto one gel, separated, blotted and probed for phospho-ERK1/2 immunoreactivity. The blot was then stripped and re-probed for DARPP32- and tyrosine hydroxylase (TH)-immunoreactivity. Digitized images of the bands were used for quantitative analysis. The control value (100%) for each protein was the average value of the bands for the wild-type intact striatum. For illustrative purposes one band for each condition from these images were used in Figure 5.