Fig. 5.
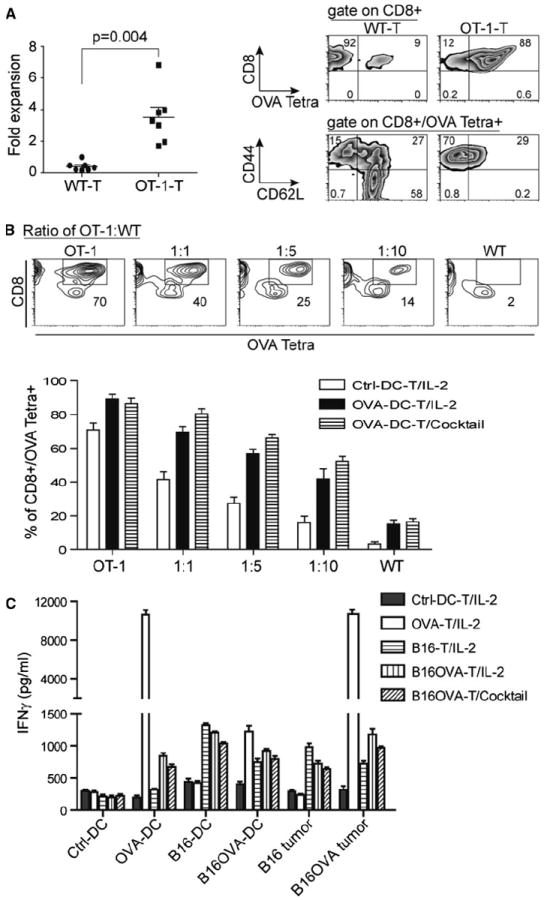
RNA–DC facilitates the antigen-specific T-cell expansion and recognition toward antigen-associated targets. a OVA RNA–DC leads to the defined expansion and enrichment of OVA-antigen-specific T cells. OVA–RNA was in vitro transcribed using pGM4Z vector harboring OVA-expressing cassette. Murine DC were electroporated with OVA RNA followed by overnight maturation; the splenic T cells from WT or OT-1 of C57bl/6 strain were co-cultured with OVA RNA–DC at a ratio of T-cell to DC, 10:1. After 6 days of ex vivo culture in the presence of IL-2 (30 IU/ml), the expansion of T cells was calculated and plotted. The percentage and phenotype of OVA Tetra+ T cells were analyzed using flow cytometry. b The specific expansion of OVA-antigen-specific T cells from a mixed population. DC was cocultured with T cells from OT-1 or WT mice or their mixture at a decreased ratio of OT-1 to WT for 6 days. Upper panel the percentage of OVA Tetra+CD8+ T cells from a representative T-cell culture with ctrl-DC was indicated in squares of each image; lower panel based upon the criteria from upper panel, the percentage of OVA Tetra+CD8+ T cells co-cultured with ctrl-DC or OVA RNA–DC in the presence of IL-2 or cocktail was shown, and the data were plotted as average ± SD from 3 independent experiments. c The specific IFNγ induction occurs only in antigen-associated targets. The different types of RNA–DC-expanded T cells were used as effector cells, and the antigen-associated targets were from B16F10 and B16F10OVA tumor lines, and from DC electroporated with RNA (OVA, B16F10, and B16F10OVA) as surrogate targets. Except the last group cultured with a cocktail, the others were cultured with IL-2. After 7 days expansion, T cells (2.5 × 105/well) were co-cultured with targets at a ratio of 10:1, and the levels of IFNγ were measured by ELISA kit
