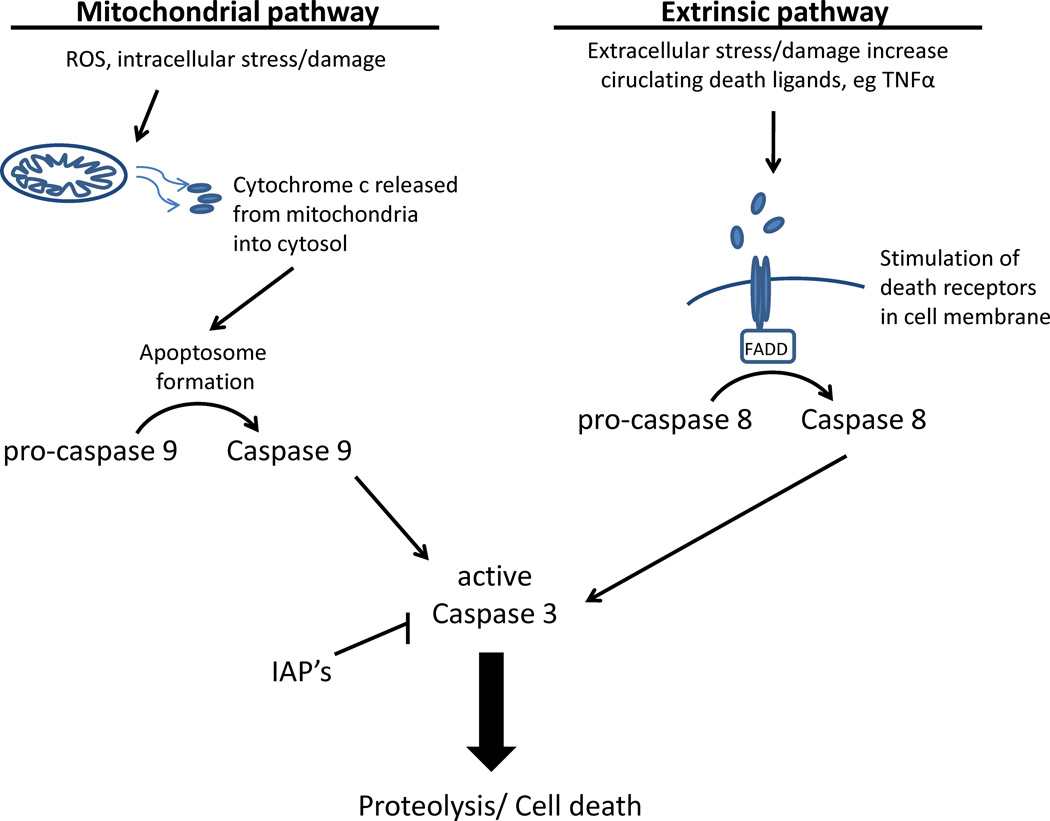
Figure 1. Overview of the intrinsic and extrinsic cell death pathways.
The intrinsic or mitochondrial pathway is triggered by cellular stress signals such as accumulation of ROS, DNA damage and relocalization or activation of proapoptotic proteins. The key event in the intrinsic pathway is mitochondrial cytochrome c release following mitochondrial membrane permeabalization. Cyt c then binds with APAF-1, facilitating formation of the apoptosome that activates procaspase-9 by proteolysis activating downstream effector caspase-3. The extrinsic pathway is initiated by activation of cell surface receptors or death receptors belonging to the tumor necrosis factor (TNF) family. These include FAS receptor (CD95), TNF receptor-1 (TNFR1), DR4, and DR5. The FAS receptor is one of the best-understood death receptors and provides a model for the extrinsic pathway. Ligands bind to these receptors and induce formation of a death-inducing signaling complex (DISC) that includes Fas associated death domain (FADD). This complex cleaves procaspase to active caspase 8, which then leads to activation of caspase-3. Once activated, caspase-3 cleaves a wide variety of substrates in the brain resulting in loss, gain, or change of function for the target protein.