Figure 4.
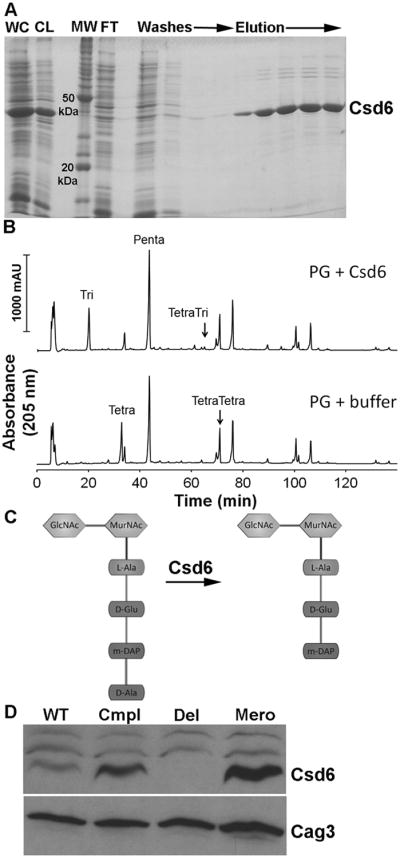
Functional analysis of Csd6 enzymatic activity and expression levels. A) SDS-PAGE gel depicting steps in the purification of oligohistidine-tagged H. pylori Csd6 protein from E. coli cells. The protein was purified using Ni-NTA resin as described in Experimental Procedures. WC, induced whole cell lysate; CL, cleared lysate; MW, molecular weight markers; FT, flow through. B) HPLC analysis of muropeptides released from purified peptidoglycan (PG, obtained from the Δcsd1csd6 mutant, strain DBH11) upon treatment with His-Csd6 or buffer followed by cellosyl digestion. Loss of monomeric tetrapeptides with formation of monomeric tripeptides in the presence of Csd6 is indicative of the protein having LD-carboxypeptidase activity. C) Schematic of the predicted activity of Csd6 based on experiment in (C) showing the substrate (tetrapeptide) and product (tripeptide). D) Antibody based detection of Csd6 in whole cell extracts prepared at equal cell density. Blots were stripped and re-probed with antisera against another periplasmic protein, Cag3, for quantitative expression analyses. One of three representative experiments is shown. WT, wildtype (LSH100); Cmpl, csd6 complement (TSH31); Del, csd6 null allele (TSH17), Mero, csd6 merodiploid (TSH35).
