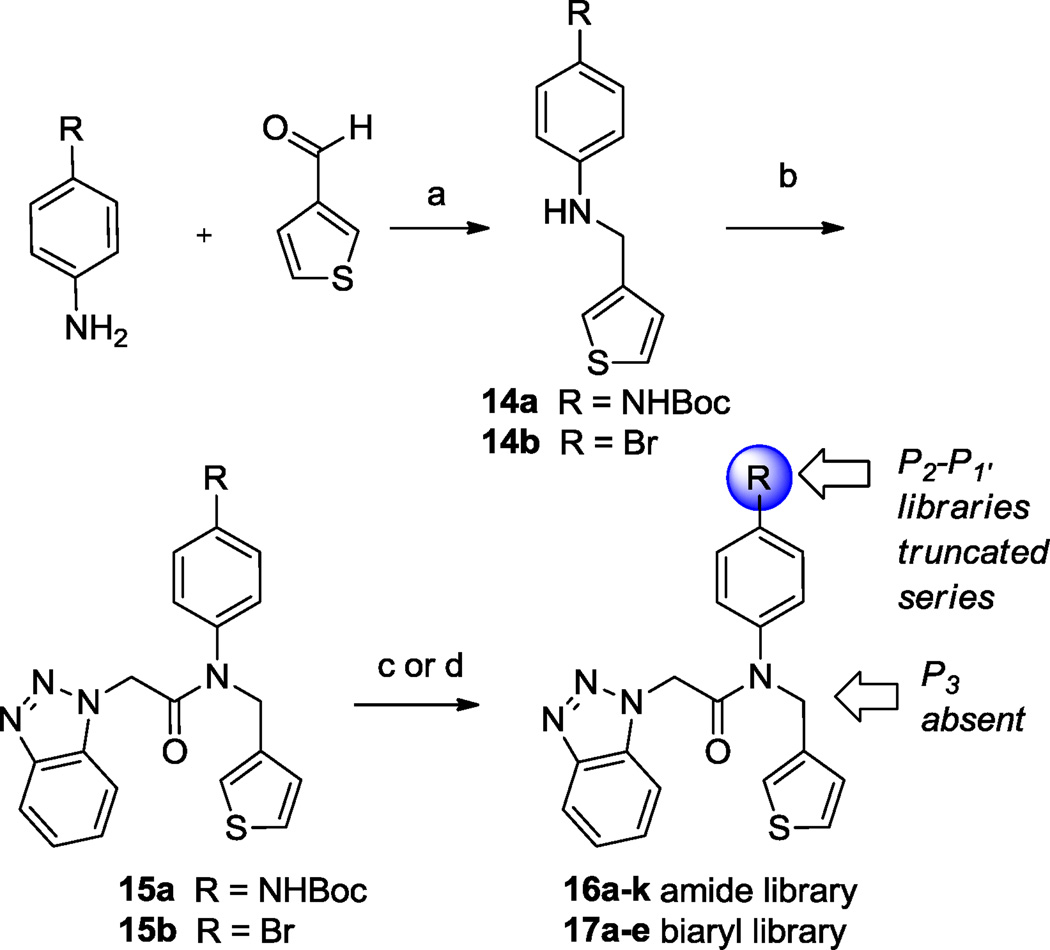
Scheme 3.
Synthesis of P2-P1, analogs 16a–k and 17a–e within truncated series. Reagents and conditions: (a) NaHB(OAc)3, DCE, rt, 80% (b) benzotriazol-1-yl-acetic acid, HATU, TEA, DMF, rt, 74% (c) (i) 15a, TFA, DCM, 95%, (ii) HATU, DIPEA, DMF, RCO2H, 65–80%; RCO2Cl or RSO2Cl, TEA, DCM, 90–95%; NaHB(OAc)3, RCHO, DCE, 45–95% (d) 15b, Ar/HetB(OH)2, 1M aq. Na2CO3, 5 mol% Pd(PPh3)4, THF, 30–65%. Final library compounds were purified by UV prep or mass-directed prep HPLC.