Figure 2.
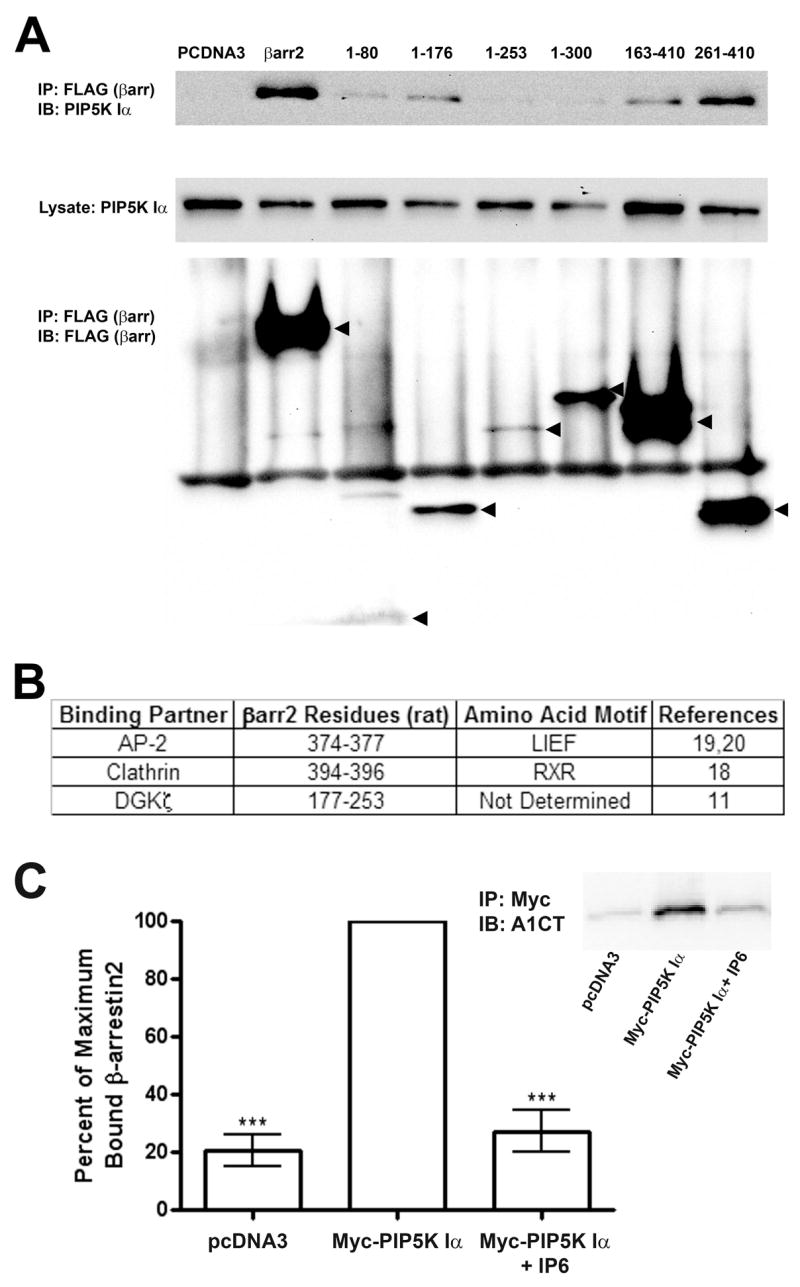
β-arrestin2 Directly Binds PIP5K Iα and is Independent of Association with DGKζ AP-2, or Clathrin. A. Western blots of endogenous PIP5K Iα (Upper and Middle panels) co-immunoprecipitated in FLAG immunoprecipitates from HEK293 cells were transfected with empty vector (pcDNA3), full-length FLAG-β-arrestin 2, or FLAG-tagged β-arrestin2 truncation mutants (Lower panel). Images shown are representative of three independent experiments. B. Table illustrating β-arrestin2 amino acid residues required for interacting with previously mapped binding partners. C. Quantification of purified recombinant β-arrestin2 co-immunoprecipitating with His6-Myc-PIP5K Iα affinity purified from HEK293 cells. Data from three independent experiments were normalized as a percentage of the maximum amount of β-arrestin2 detected by A1CT antibody for each experiment. Statistical significance was determined using a one-way ANOVA with a Bonferroni post-hoc test to correct for multiple comparisons ( **, p < 0.01, vs. PIP5K Iα immunoprecipitates without IP6). Inset: Representative A1CT immunoblot of purified β-arrestin2 co-immunoprecipited under the experimental conditions.
