FIGURE 5. Least-squares superposition of the AKR1D1-NADP+ complex (yellow), the AKR1D1-NADP+-cortisone complex (red), and the AKR1D1-NADP+-testosterone complex (green).
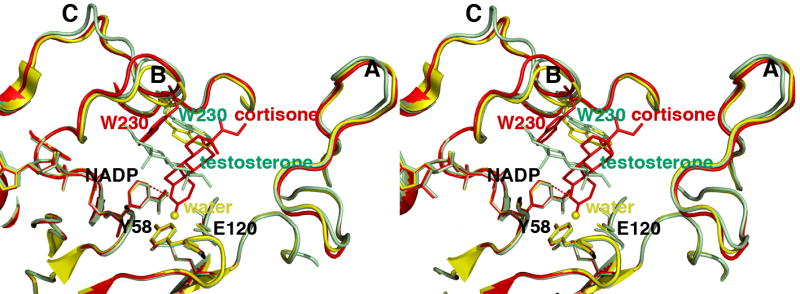
The distance of ∼3.7 Å between the anomeric carbon of NADP+ and the olefinic carbon C4 of cortisone is indicated by a red dashed line, which would represent the trajectory of hydride transfer from NADPH. The position of the water molecule in the unliganded structure is indicated by a yellow sphere. Loops A, B, and C are labeled; note that loop B and W230 in particular must undergo a significant conformational change to accommodate productive substrate binding as represented by cortisone (red). The indole ring of W230 remains in its substrate free conformation (yellow) to accommodate the nonproductive binding mode of testosterone (green).
