Abstract
In functional MRI studies, repetition suppression refers to the reduction of hemodynamic activation to repeated stimulus presentation. For example, the repeated presentation of a face reduces the hemodynamic response evoked by faces in the fusiform gyrus. The neural events that underlie repetition suppression are not well understood. Indeed, in contrast to the hemodynamic response, the face-specific N200 recorded from subdural electrodes on the ventral occipitotemporal cortex, primarily along the fusiform gyrus, has been reported to be insensitive to face-identity repetition. We have previously described a face-specific broadband gamma (30–100 Hz) response at ventral face-specific N200 sites that is functionally dissociable from the N200. In this study, we investigate whether gamma and other components of the electroencephalogram spectrum are affected by face-identity repetition independently of the N200. Participants viewed sequentially presented identical faces. At sites on and around the fusiform gyrus, we found that face repetition modulated alpha (8–12 Hz), low-gamma (30–60 Hz), and high-gamma (60–100 Hz) synchrony, but not the N200. These findings provide evidence of a spatially co-localized progression of face processing. Whereas the N200 reflects an initial obligatory response that is less sensitive to face-identity repetition, the subsequent spectral fluctuations reflect more elaborative face processing and are thus sensitive to face novelty. It is notable that the observed modulations were different for different frequency bands. We observed repetition suppression of broadband gamma, but repetition enhancement of alpha synchrony. This difference is discussed with regard to an existing model of repetition suppression and behavioral repetition priming.
Keywords: ECoG, ERP, face, alpha, gamma, vision, repetition suppression
INTRODUCTION
The brain’s response to a stimulus is often reduced by its repeated presentation—a prevalent feature of neural processing that has been described as the simplest form of learning [Groves and Thompson, 1970; Thorpe, 1956]. This phenomenon has different names in different literatures; within fMRI, it is commonly referred to as repetition suppression (habituation and adaptation are also used). Repetition suppression can be observed in the brain’s face perception system, where the face-specific hemodynamic response of the fusiform gyrus (FG) is diminished upon repeated presentations of the same face [Andrews and Ewbank, 2004; Eger et al., 2004; Gauthier et al., 2000; Gilaie-Dotan and Malach, 2007; Henson, 2000; Winston et al., 2004]. Similarly, electro/magneto-encephalographic recordings made from the scalp have shown suppression within the face processing system to repeated face-identity repetition (i.e., repetition of the same individual) as indexed by amplitude changes in the N170/M170 [Caharel et al., 2009; Ewbank et al., 2008; Henson et al., 2003; Jacques et al., 2007] event-related potentials (ERPs). However, these potentials represent volume-conducted signals from several brain areas. Therefore, it is unclear whether any observed effect of stimulus-repetition reflects changes in local neural behavior at face-selective locations of cortex, or rather, influence from neural responses across the brain. Like the scalp-recorded N170, subdurally recorded face-selective ERPs respond more strongly to faces than to all other tested stimulus categories [Allison et al., 1994, 1999; Engell and McCarthy, 2010, 2011; Jonas et al., 2012; Lachaux et al., 2005; Parvizi et al., 2012; Privman et al., 2007; Puce et al., 1997]. The peak of these face-selective ERPs is often observed at ~200 ms post face-onset [Allison et al., 1994, 1999; Engell and McCarthy, 2010, 2011; Puce et al., 1997] and will thus be referred to as the face-N200 [cf. Allison et al., 1999].
To investigate whether face-repetition affects changes in local neural behavior at face-selective locations of cortex requires intracranial recording. To date, only one such study has been conducted that investigates face-identity repetition. Puce et al. [1999] found no effect of repetition on the face-N200.
We have previously shown that the EEG spectrum recorded from ventral face-specific N200 sites includes a face-specific broadband gamma (30–100 Hz) response (face-γ) that is functionally dissociable from the face-N200 [Engell and McCarthy, 2010, 2011]. For instance, face-γ is sensitive to the featural complexity of a face (e.g., color “cartoon” faces versus simple line drawn faces), whereas the face-N200 is largely unaffected by these differences [Engell and McCarthy, 2011]. We have thus proposed that the ventral face-specific N200 represents an initial obligatory response to a face, whereas face-γ represents more elaborative subsequent processing such as identity discrimination [Engell and McCarthy, 2010, 2011]. We therefore expect identity repetition effects to be evident in the latter, but not the former.
In this report, we investigated the effect of face-identity repetition on spectral power recorded subdurally from occipitotemporal sites from which the face-specific N200 has been reported [Puce et al., 1999].
MATERIALS AND METHODS
EEG Acquisition
Recordings were analyzed from 16 electrodes (nine in the right hemisphere, seven in the left hemisphere) collected from 10 patients (age range: 25–49 years, median age = 39, five females, five males) with medically intractable epilepsy who were being evaluated for possible surgery by the Yale Comprehensive Epilepsy Center [Spencer et al., 1982]. A complete description of these patients can be found in Allison et al. [1999]. These electrodes are a subset of the 28 electrodes examined by Puce et al. [1999] in their study of repetition sensitivity of the face-specific N200. Data from 12 electrodes used by Puce et al. [1999] were initially recorded as short stimulus-locked epochs rather than as continuous EEG, and were thus not suitable for time-frequency analysis.
Strips or grids of stainless steel electrodes (2.2 mm surface diameter) were placed subdurally on the cortical surface. The electrode placements were determined individually for each patient according to their clinical histories, and thus electrode locations varied across individuals. The studies reported here were among several sensory and cognitive experiments in which each subject participated, typically 4–8 days following implantation of electrodes. At the time of participation, medication levels to control seizures and post-operative pain varied across patients. The EEG experiments were not conducted immediately before or after seizures nor were any of our sites of interest revealed to be in epileptogenic cortex. The EEG protocol was approved by the IRB of the Yale University School of Medicine. All participants provided informed consent.
Local field potentials were recorded referentially from 64 electrode sites and amplified with a common reference (either the mastoid or a small post electrode in the patient’s skull) using an SA Instruments EEG amplifier system with a 0.1–100 Hz bandpass filter. The EEG signal was continuously acquired and digitized at 250 Hz. The digitized signal was written to disk along with a digital code that marked the onset of each stimulus.
Stimuli and Procedure
Stimulus presentation was computer controlled and displayed on a CRT monitor (640 × 480 pixels) positioned on a table over the patient’s bed. The viewing distance was adjusted for patient comfort. Stimuli included 40 color images of novel faces. Patients were asked to view each face as it was presented in the center of the display, but were not required to make any overt responses. Faces were presented at intervals of 2 s and remained onscreen for 500 ms. Faces were grouped into sets in which the same face would appear eight times consecutively followed by the next set of eight presentations of a new face, and so on for a total of 40 sets of faces.
Event-Related Potential Analysis
Puce et al. [1999] conducted a full analysis of repetition effects upon face-specific ERPs. Because we studied a subset of the electrodes used in their original analyses, we repeated the analysis here to ensure the results were consistent. Our analyses were performed using custom MAT-LAB (The Mathworks) functions. Residual line noise (60 Hz) filtering was performed in Matlab using a fifth order Butterworth filter that was applied in a temporally symmetric manner to avoid introducing phase shifts. Baseline adjusted ERPs were created by signal averaging the EEG signal across trials for each experimental condition and subtracting from each time-point the average of a 100 ms pre-stimulus epoch. A temporally symmetric smoothing kernel with a total length of five time-points (from −2 to +2 time points) was convolved with the average ERP waveforms prior to amplitude and latency measurements of P150, N200, and P290.
A subset of the 10 patients had more than one face-N200 site. We therefore averaged across face-N200 electrodes within each participant prior to statistical analyses, effectively reducing our sample size from 16 to 10. The same procedure was applied to the time-frequency analysis described below. In their report of these data, Puce et al. [1999] treated all face-N200 sites, regardless of whether they were recorded from within the same patient, as independent samples. To confirm that our ERP results were consistent with the prior report, our analyses were run a second time treating all 16 electrodes as independent samples. The results of these two approaches were qualitatively identical. That is, any and all statistically significant effects were present in both analyses.
Independent one-way ANOVAs were used to evaluate whether the ERP amplitudes or latencies varied as a function of identity repetition. Sphericity violations were addressed by adjusting the degrees of freedom using the Greehouse–Geisser method. These corrections did not qualitatively affect the results and so, unless otherwise noted, we only report the unadjusted degrees of freedom and associated P-value.
Time-Frequency Analysis
Time-frequency power spectra were estimated using EEGLAB v11 [Delorme and Makeig, 2004] and MATLAB v7.9 (The Mathworks). Seventy-six linearly spaced frequencies between 9 and 125 Hz were estimated using Morelet wavelet analysis based on 3 cycles at the lowest frequency increasing to 20.75 cycles at the highest frequency. Spectral power estimates were averaged to create spectral power waves representing power within four frequency bands (alpha: 8–12 Hz, beta: 12–30 Hz, low-gamma: 30–60 Hz, and high-gamma: 60–100 Hz). The frequency range for gamma was selected on the basis of prior reports in the animal [Singer and Gray, 1995] and human [Engell and McCarthy, 2010, 2011; Engell et al., 2012; Fisch et al., 2009; Lachaux et al., 2005; Tallon-Baudry and Bertrand, 1999; Tsuchiya et al., 2008] literatures, which have defined 30 Hz as the lower bound of the gamma band. Human intracranial studies have reported an upper bound for gamma between 70 and 200 Hz. The bandpass used in our studies imposed a 100 Hz (−3 db) upper limit on the EEG signal, and so we restricted the upper range of the gamma band to 100 Hz. There are some reports of heterogeneity with the gamma band as reflected in dissociable responses of “low-gamma” from “high-gamma” [cf. Crone et al., 2011]. We therefore investigated these bands separately.
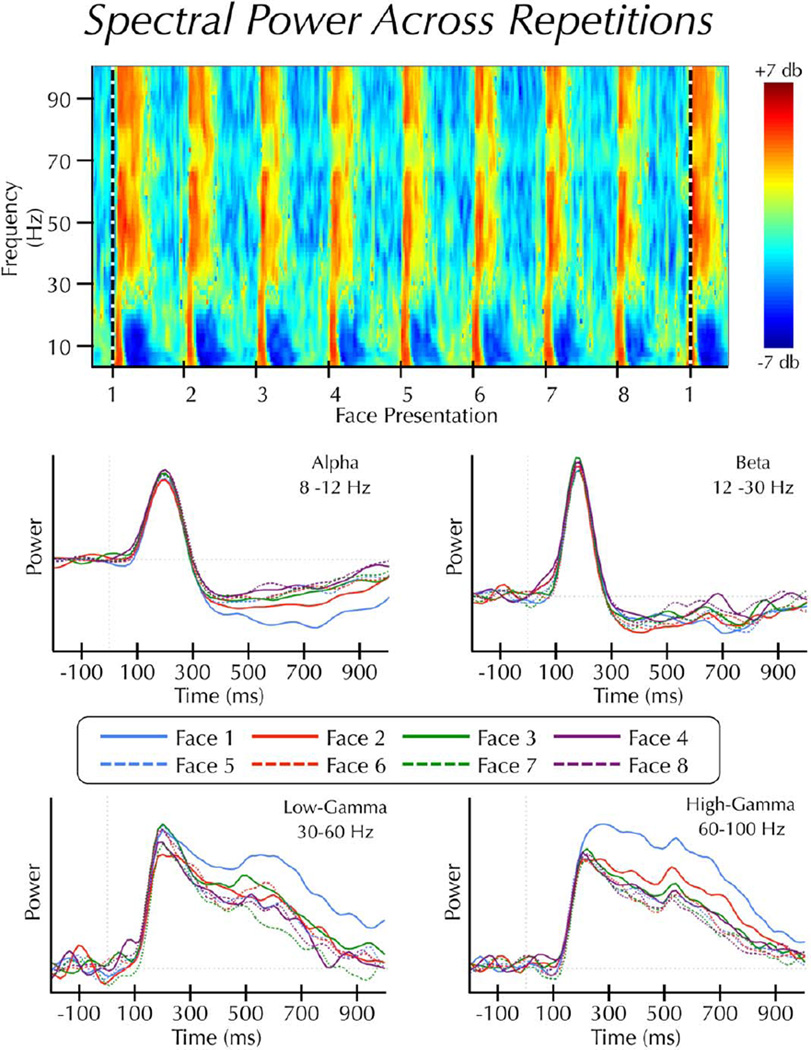
We further simplified the data by estimating the area under the curve (AUC) of the spectral power waves within each of two epochs: early (100–300 ms) and late (300–1,000 ms). These epochs were chosen to capture the dynamic nature of the changes in the power spectrum, particularly within the alpha band. In the low-frequency range, stimuli induced an increase in synchrony beginning at approximately 100 ms post-stimulus. This increase was immediately followed by a period of sustained desynchronization that began at approximately 300 ms (see Fig.2).
Figure 2.
Spectral power across repetitions. (Top panel) Average time-frequency results displayed as log power changes (db) from a 3 s pre-block baseline epoch. Face presentations are separated by 2 s. The horizontal dashed line indicates the start of a new face identity set. Note: this panel is for illustrative purposes only. Statistical analyses were not performed on spectral estimates relative to a pre-block baseline epoch. (Bottom panel) Average power estimates across the first 1 s of each trial within the alpha, beta, low-gamma, and high-gamma bands. Note: this figure displays continuous power changes over time, whereas the analysis was performed on area under the curve estimates from these waveforms.
Face-identity repetition effects were tested using independent one-way ANOVAs for each epoch and frequency band. Corrections for sphericity violations were the same as those used for the ERP analysis. For frequency bands and epochs that showed significant effects, we used a least-squares approach to fit trend lines to the data across repetitions.
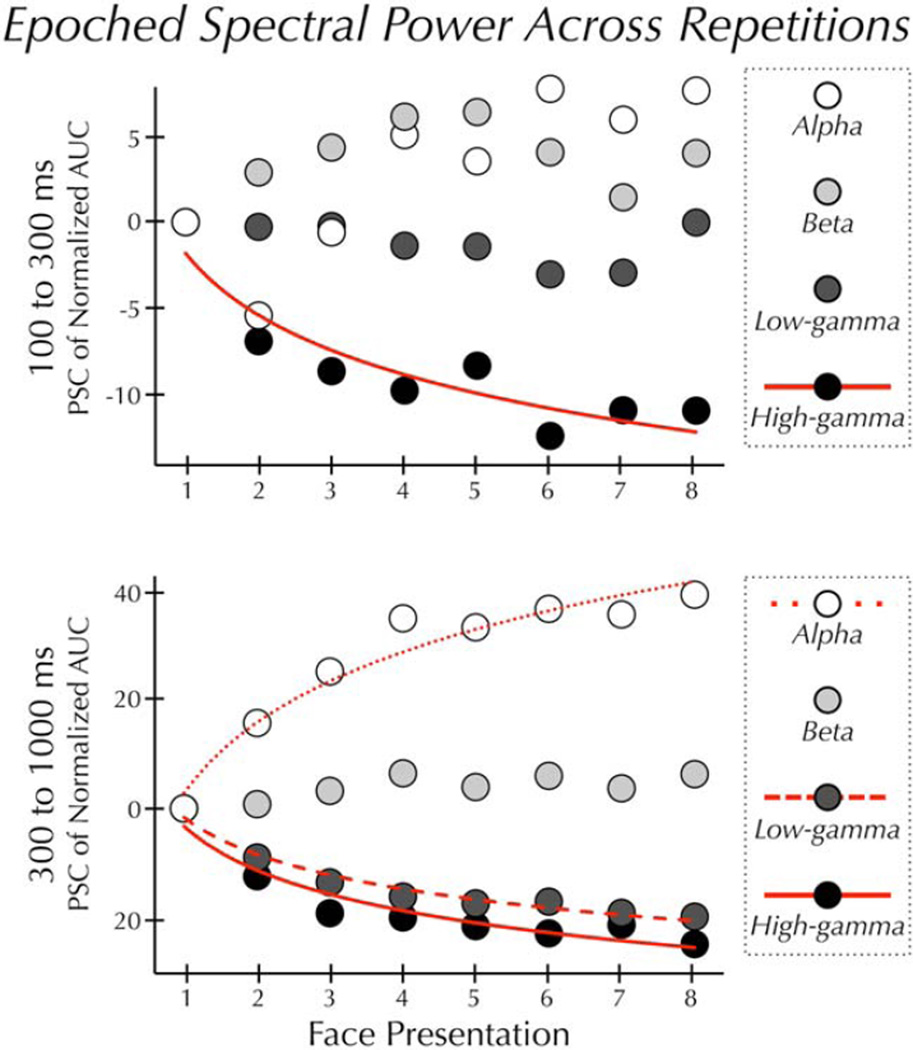
Prior to plotting (see Fig. 3), the AUC estimates were normalized to a range of 0–1; Xi.normal = Xi − Xmin/Xmax − Xmin, where, within a given Xmax − Xmin, where, within a given frequency band and given epoch, Xi.normal represents the new normalized AUC estimate for a particular channel, Xi represents the original AUC estimate at that channel, and Xmin and Xmax represent the minimum and maximum AUC estimate across all channels, respectively.
Figure 3.
Epoched spectral power across repetitions. Mean power at each of eight sequential presentations of the same face. Prior to plotting, the AUC data were normalized between 0 and 1 (see Methods). Within each frequency band, AUC at each trial is plotted relative to the AUC induced by the first presentation of the face. (Top panel) In the early epoch power in the high-gamma band varied significantly and was best described by a decreasing power-law function (solid line). Power in the alpha, beta, and low-gamma bands did not significantly vary across repetitions. (Bottom panel) In the late epoch power in the alpha band varied significantly and was best described by an increasing logarithmic function (dotted line). Power in the low- and high-gamma bands also varied significantly. These changes in power were best described by a decreasing power-law function (dashed and solid line). Power in the beta band did not significantly vary across repetitions.
RESULTS
Event-Related Potentials
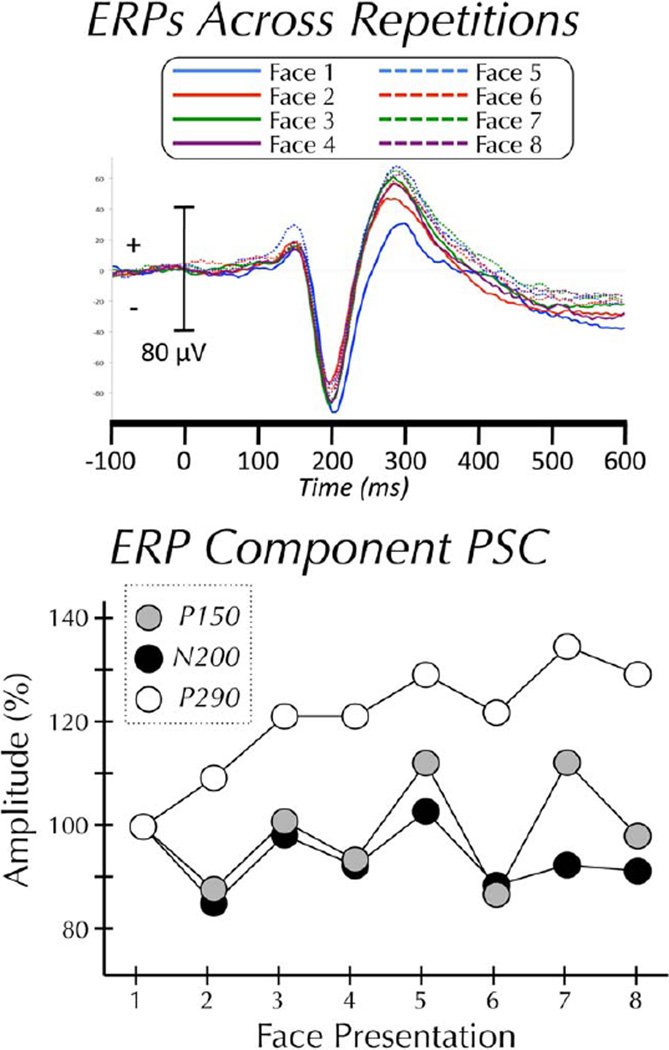
The P150, N200, and P290 ERP components were examined (Fig. 1). The amplitude of each of the components did not vary significantly across repetitions (Ps>0.05). Although it did not reach significance, the amplitude of the P290 did increase with repetition. The latency of each of the components did not vary significantly across repetitions (Ps>0.05). To ensure that subtle latency shifts were not being obscured by smoothing the signal (see Event-Related Potential Analysis section of Methods), all analyses were run a second time on unsmoothed data. This analysis found no significant effect of latency for the P150, N200, or P290.
Figure 1.
ERPs across repetitions. (Top panel) Grand-average ERP (N = 10) to each of eight repetitions of the same face. (Bottom panel) Mean amplitude of P150, N200, and P290 components at each of eight sequential presentations of the same face.
Although there were no progressive effects of repetition, visual inspection of the trial-to-trial change in N200 amplitude (see Fig. 1) shows a decrease between trials 1 and trial 2. We therefore performed post hoc t-tests of the amplitude change between each trial and its preceding trial. Using a liberal uncorrected threshold of P < 0.05 we found two trial-to-trial differences in N200 amplitude; trial 2 was significantly smaller than trial 1 and trial 3 was significantly larger than trial 2. There were no significant trial-to-trial differences for the P150 or the P290.
Time-Frequency
Face repetition resulted in different effects across epochs and frequency bands (Fig. 2). In the early epoch, there was no significant effect of repetition in the beta or low-gamma bands (all Ps> 0.05), and a marginally significant effect in the alpha band (F(7,63) = 2.89, P = 0.011, corrected for sphericity P = 0.052). High-gamma power varied (F(7,63) = 9.03, P < 0.001) as a power-law function of repetition time (R2 = 0.85) (Fig. 3).
In the late epoch, there was no significant effect of repetition in the beta band. Alpha power varied (F(7,63) = 29.76, P<0.001) as a logarithmic function of repetition time (R2 = 0.95) (Fig. 3). Low- and high-gamma varied significantly (low-γ: F(7,63) = 17.57, P < 0.001; high-γ: F(7,63) = 16.19, P = 0.001) as a power-law function of repetition time (low-γ: R2 = 0.97; high-γ: R2 = 0.92) (Fig. 3). The RS in the high-γ band was more pronounced in the late than the early epoch.
DISCUSSION
EEG recordings made from the cortical surface of the FG and surrounding cortices show co-localized functionally dissociated evoked (face-N200) and induced (event-related spectral power changes) responses, particularly in the gamma band [Engell and McCarthy, 2010, 2011]. Here, we report that this dissociation includes face-identity repetition effects. Whereas successive repetitions of the same face-identity lead to a progressive and monotonic reduction of induced γ-power, there is no such change in the amplitude or latency of the face-N200. There are several possibilities for the lack of effect on the N200 in this study, including our particular experimental parameters (see below), which preclude us from concluding that the N200 is insensitive to identity repetition per se. However, we have unequivocally shown that the co-localized face-induced change in γ-power is significantly more sensitive to such repetition.
Our finding that the P150 and face-specific N200 show no progressive reduction in amplitude to face-identity repetition confirms in this sample subset what Puce et al. [1999] reported from the full sample of these same data. Puce and colleagues also reported that P290 increased in amplitude with repetition. We observed the same mean P290 amplitude increase; however, this increase did not reach statistical significance in this sample subset. In striking contrast to the relative insensitivity of the N200, we observed strong modulation of the EEG frequency spectrum as a function of repeated face-identity. Repetition resulted in a progressive increase in low-frequency α-power and a progressive decrease in high-frequency γ-power. This finding has important implications for both our understanding of face-processing and repetition suppression.
We have proposed that the face-specific N200 generated in the FG is an obligatory response to faces, which accounts for its general insensitivity when challenged with cognitive and perceptual manipulations [Allison et al., 1999; Engell and McCarthy, 2010, 2011; Puce et al., 1999]. In contrast, changes in spectral power reflect more elaborative processing. Although the N200 was not entirely unaffected by repetition, it was not affected in a consistent or progressive manner. The amplitude of the N200 to the second presentation of a given face-identity was significantly smaller than to the first presentation. However, this was immediately followed by a significant increase (trial 2 to trial 3) in amplitude.
The peak of the face-N200 is often observed at ~200 ms post-stimulus. However, this peak latency varies considerably across studies and patients and has been observed earlier than 200 ms [Jonas et al., 2012; Lachaux et al., 2005; Parvizi et al., 2012; Privman et al., 2007]. Might this latency difference represent a meaningful functional difference and, if so, might this mean that repetition-suppression can affect face-N170 sites but not face-N200 sites? Although this study cannot offer strong evidence against this possibility, we believe it is unlikely. Unpublished observations from our laboratory’s 20 years of face-selective recordings show that most of this variability is observed between-patients, rather than between face-selective sites within-patients. This suggests that the latency differences reflect individual differences across patients, rather than functionally meaningful differences across cortical locations. Moreover, in this study we observed no qualitative difference in the effect of repetition-suppression on three (of 16) face-selective sites that peaked ≤180 ms post-stimulus.
In contrast to the face-N200, the repetition suppression of gamma power was continuous and monotonic. These results are consistent with effects of stimulus repetition on broadband γ-power seen in the macaque [De Baene and Vogels, 2010] and in the human using scalp recorded EEG [Gruber and Müller, 2002, 2005] and MEG [Friese et al., 2012a, b]. However, a prior intracranial EEG study found no modulation of the induced gamma response [Privman et al., 2011]. How can we reconcile those findings with this report?
Given our interpretation of our results the first, and perhaps most critical, difference between the two studies is that Privman et al. [2011] investigated face-category repetition, whereas we investigated face-identity repetition. Indeed, our proposal that the face-induced gamma response reflects elaborative processing of the face’s identity is consistent with the results of both reports. In this report, we show that repetition of the same identity results in reduced gamma power, while Privman et al. [2011] showed that repeating faces with different identities did not reduce gamma power. However, we acknowledge that this data do not speak fully to this interpretation because our study does not include a face-category repetition condition.
Second, we note that the analysis used in the prior report of Privman et al. [2011] focused on gamma power at frequencies below 70 Hz within a brief window after stimulus-repetition. Our results show that the effect of face repetition is most prominent in the sustained response at higher frequencies (60–100 Hz).
Last, the stimulus onset asynchrony (SOA), the latency at which repeated stimuli are presented, was different across the two studies. Privman and colleagues found that the face-category repetition ERP effect was sensitive to SOA, such that it was observed at an SOA of 200 ms, but not an SOA of 400 ms. In light of this, an important limitation of this study is that repeated faces were displayed using a long SOA of 2,000 ms. It is thus possible that a shorter SOA would have revealed face-identity repetition sensitivity of the face-N200. Although this precludes us from making a strong declaration as to whether the face-N200 can be modulated by face-identity repetition, the results unequivocally demonstrate a dissociation between the sensitivity of this ERP and the induced gamma response. At minimum, the gamma response is significantly more sensitive to face-identity repetition than is the N200.
Repetition suppression is a counterintuitive phenomenon given that repetition facilitates cognitive and perceptual processing [cf. Schacter and Buckner, 1998], an effect often referred to as repetition priming. A number of possible mechanisms have been proposed for why a reduction in the neural response leads to facilitated behavior. A full description of these competing hypotheses is beyond the scope of this report (for a recent review see Gotts et al. [2012]), but one, “neural synchrony,” is particularly relevant to these results. This hypothesis posits that behavioral facilitation is a consequence of an increase in synchronous activity, despite a decrease in the overall firing rate of neurons [Gotts, 2003]. In support of this hypothesis, MEG studies have found that repeated stimulus presentations increase local [Gilbert et al., 2010] and inter-regional coherence [Ghuman et al., 2008] in the alpha band. Our study does not include a behavior component, and thus does not speak directly to the repetition suppression/priming paradox. However, our finding of local alpha band repetition enhancement (manifested as a progressive decrease of face-induced desynchronization) is consistent with the neural synchrony hypothesis and the first such evidence with the spatiotemporal resolution of intracranial EEG.
Notably, while we observed repetition enhancement of a-power, we observed repetition suppression of broadband g-power. The latter is consistent with the effect of repetition on the hemodynamic response [Andrews and Ewbank, 2004; Eger et al., 2004; Gauthier et al., 2000; Gilaie-Dotan and Malach, 2007; Henson, 2000; Winston et al., 2004]. These results fit with the observation that the hemodynamic response is positively correlated with gamma-band power [Hermes et al., 2012; Koch et al., 2009; Lachaux et al., 2007; Mukamel et al., 2005; Niessing et al., 2005; Ojemann et al., 2010; Scheeringa et al., 2011], while negatively correlated with alpha-band power [Hermes et al., 2011; Mukamel et al., 2005; Niessing et al., 2005; Scheeringa et al., 2011]. Unlike the alpha synchrony that is thought to support information transactions between regions, broadband gamma likely reflects local neuronal responses. It has been correlated with spiking activity as measured with multi-unit recordings in the human [Manning et al., 2009] and in the macaque [Rasch et al., 2008; Ray et al., 2008; Ray and Maunsell, 2011].
CONCLUSIONS
In summary, these results show progressive modulation of α-power and broadband γ-power as a function of face repetition with no concomitant effect in the face-specific N200. This suggests that multiple levels of face processing are spatially co-localized in ventral occipitotemporal cortex. Additionally, the observed progressive increase in α-power is consistent with the neural synchrony model of repetition suppression/priming.
ACKNOWLEDGMENTS
The authors thank Drs. Truett Allison, Aina Puce, and Dennis D. Spencer, and Mr. Joseph Jasiorkowski for their help in acquiring the intracranial EEG data reported here.
Contract grant sponsor: National Institute of Mental Health; Contract grant numbers: MH-093986 (A.D.E.) and MH-05286 (G.M.).
REFERENCES
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD. Face recognition in human extrastriate cortex. J Neurophysiol. 1994;71:821. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. Neuroimage. 2004;23:905–913. doi: 10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Caharel S, d’Arripe O, Ramon M, Jacques C, Rossion B. Early adaptation to repeated unfamiliar faces across viewpoint changes in the right hemisphere: Evidence from the N170 ERP component. Neuropsychologia. 2009;47:639–643. doi: 10.1016/j.neuropsychologia.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: Searching high and low. Int J Psychophysiol. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baene W, Vogels R. Effects of adaptation on the stimulus selectivity of macaque inferior temporal spiking activity and local field potentials. Cereb Cortex. 2010;20:2145–2165. doi: 10.1093/cercor/bhp277. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Eger E, Schyns PG, Kleinschmidt A. Scale invariant adaptation in fusiform face-responsive regions. Neuroimage. 2004;22:232. doi: 10.1016/j.neuroimage.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Engell AD, Huettel S, McCarthy G. The fMRI BOLD signal tracks electrophysiological spectral perturbations, not event-related potentials. Neuroimage. 2012;59:2600–2606. doi: 10.1016/j.neuroimage.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, McCarthy G. Selective attention modulates face-specific induced gamma oscillations recorded from ventral occipitotemporal cortex. J Neurosci. 2010;30:8780–8786. doi: 10.1523/JNEUROSCI.1575-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, McCarthy G. The relationship of gamma oscillations and face-specific ERPs recorded subdurally from occipitotemporal cortex. Cereb Cortex. 2011;21:1213–1221. doi: 10.1093/cercor/bhq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Smith WA, Hancock ER, Andrews TJ. The M170 reflects a viewpoint-dependent representation for both familiar and unfamiliar faces. Cereb Cortex. 2008;18:364–370. doi: 10.1093/cercor/bhm060. [DOI] [PubMed] [Google Scholar]
- Fisch L, Privman E, Ramot M, Harel M, Nir Y, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Fried I, Malach R. Neural “ignition”: Enhanced activation linked to perceptual awareness in human ventral stream visual cortex. Neuron. 2009;64:562–574. doi: 10.1016/j.neuron.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese U, Rahm B, Hassler U, Kaiser J, Gruber T. Repetition suppression and effects of familiarity on blood oxygenation level dependent signal and gamma-band activity. Neuroreport. 2012a;23:757–761. doi: 10.1097/WNR.0b013e328356b173. [DOI] [PubMed] [Google Scholar]
- Friese U, Supp GG, Hipp JF, Engel AK, Gruber T. Oscillatory MEG gamma band activity dissociates perceptual and conceptual aspects of visual object processing: A combined repetition/conceptual priming study. Neuroimage. 2012b;59:861–871. doi: 10.1016/j.neuroimage.2011.07.073. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. J Cogn Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Proc Natl Acad Sci USA. 2008;105:8405–8409. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Malach R. Sub-exemplar shape tuning in human face-related areas. Cereb Cortex. 2007;17:325–338. doi: 10.1093/cercor/bhj150. [DOI] [PubMed] [Google Scholar]
- Gilbert JR, Gotts SJ, Carver FW, Martin A. Object repetition leads to local increases in the temporal coordination of neural responses. Front Hum Neurosci. 2010;4:1–12. doi: 10.3389/fnhum.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ. Thesis. Pittsburgh, PA: Carnegie Mellon University; 2003. Mechanisms underlying enhanced processing efficiency in neural systems. [Google Scholar]
- Gotts SJ, Chow CC, Martin A. Repetition priming and repetition suppression: Multiple mechanisms in need of testing. Cogn Neurosci. 2012;3:250–259. doi: 10.1080/17588928.2012.697054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychol Rev. 1970;77:419. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM. Effects of picture repetition on induced gamma band responses, evoked potentials, and phase synchrony in the human EEG. Cogn Brain Res. 2002;13:377–392. doi: 10.1016/s0926-6410(01)00130-6. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb Cortex. 2005;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- Henson R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson RN, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cereb Cortex. 2003;13:793. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Hum Brain Mapp. 2012;33:1689–1699. doi: 10.1002/hbm.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques C, d’Arripe O, Rossion B. The time course of the inversion effect during individual face discrimination. J Vis. 2007;7:3. doi: 10.1167/7.8.3. [DOI] [PubMed] [Google Scholar]
- Jonas J, Descoins M, Koessler L, Colnat-Coulbois S, Sauvee M, Guye M, Vignal JP, Vespignani H, Rossion B, Maillard L. Focal electrical intracerebral stimulation of a face-sensitive area causes transient prosopagnosia. Neuroscience. 2012;222:281–288. doi: 10.1016/j.neuroscience.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Koch SP, Werner P, Steinbrink J, Fries P, Obrig H. Stimulus-induced and state-dependent sustained gamma activity is tightly coupled to the hemodynamic response in humans. J Neurosci. 2009;29:13962–13970. doi: 10.1523/JNEUROSCI.1402-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, Kahane P, Renault B. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M. Relationship between task-related gamma oscillations and BOLD signal: New insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007;28:1368–1375. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Corina DP, Corrigan N, Schoenfield-McNeill J, Poliakov A, Zamora L, Zanos S. Neuronal correlates of functional magnetic resonance imaging in human temporal cortex. Brain. 2010;133:46–59. doi: 10.1093/brain/awp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Jacques C, Foster BL, Withoft N, Rangarajan V, Weiner KS, Grill-Spector K. Electrical stimulation of human fusiform face-selective regions distorts face perception. J Neurosci. 2012;32:14915–14920. doi: 10.1523/JNEUROSCI.2609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privman E, Nir Y, Kramer U, Kipervasser S, Andelman F, Neufeld MY, Mukamel R, Yeshurun Y, Fried I, Malach R. Enhanced category tuning revealed by intracranial electroencephalograms in high-order human visual areas. J Neurosci. 2007;27:6234–6242. doi: 10.1523/JNEUROSCI.4627-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privman E, Fisch L, Neufeld MY, Kramer U, Kipervasser S, Andelman F, Yeshurun Y, Fried I, Malach R. Antagonistic relationship between gamma power and visual evoked potentials revealed in human visual cortex. Cereb Cortex. 2011;21:616–624. doi: 10.1093/cercor/bhq128. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Spencer SS, Spencer DD, McCarthy G. Comparison of cortical activation evoked by faces measured by intracranial field potentials and functional MRI: Two case studies. Hum Brain Mapp. 1997;5:298–305. doi: 10.1002/(SICI)1097-0193(1997)5:4<298::AID-HBM16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cereb Cortex. 1999;9:445. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- Rasch MJ, Gretton A, Murayama Y, Maass W, Logothetis NK. Inferring spike trains from local field potentials. J Neurophysiol. 2008;99:1461–1476. doi: 10.1152/jn.00919.2007. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Boil. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Review. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson K-M, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen M. Neuronal dynamics underlying high-and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD, Williamson PD, Mattson RH. The localizing value of depth electroencephalography in 32 patients with refractory epilepsy. Ann Neurol. 1982;12:248–253. doi: 10.1002/ana.410120306. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Thorpe WH. Learning and instinct in animals. Cambridge, MA USA: Harvard University Press; 1956. [Google Scholar]
- Tsuchiya N, Kawasaki H, Oya H, Howard MA, Adolphs R. Decoding face information in time, frequency and space from direct intracranial recordings of the human brain. PLoS One. 2008;3:e3892. doi: 10.1371/journal.pone.0003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Henson RN, Fine-Goulden MR, Dolan RJ. fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. J Neurophysiol. 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]