Figure 2. Oligomerization of BAK by the isolated p53 DNA binding domain (102–292), C. elegans p53 Cep-1 (220–420), and tumor-derived mutants of p53.
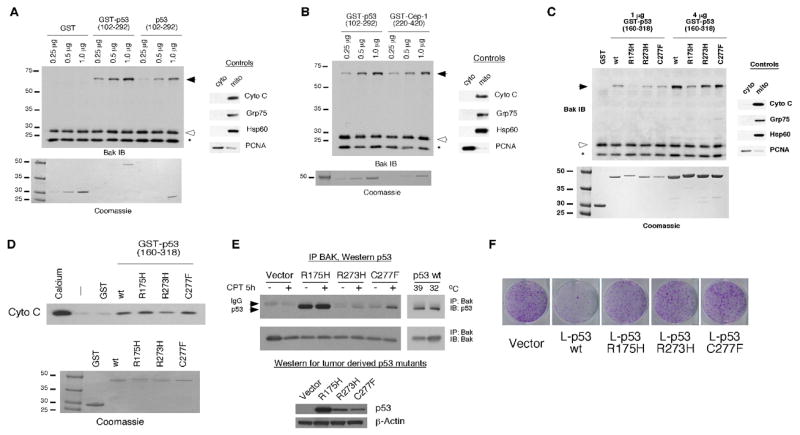
A) 20 ug of mitochondria purified from H1299 cells were incubated with 0.25, 0.5, and 1.0 ug of recombinant GST, GST-p53 (102–292), and p53 DNA binding domain alone (102–292). The purity of mitochondrial preparations was detected by immunoblotting with antisera to controls, cytochrome c, GRP75 and Hsp60 (mitochondrial proteins) as well as PCNA (cytosolic/nuclear protein) (right panel). To ensure equal input of recombinant proteins, 0.25, 0.5, and 1.0 ug of protein were subjected to SDS-PAGE and Coomassie staining (bottom panel).
B) The indicated concentrations of recombinant GST-p53 and GST-Cep-1 (0.25, 0.5, and 1.0 ug) were incubated with 20 ug of mitochondria purified from H1299 cells and subsequently cross-linked with BMH. BAK oligomers following BMH crosslinking are depicted in the left panel; the purity of mitochondrial preparations was confirmed by immunoblotting as described in 2A. Equal input of recombinant proteins was assessed by SDS-PAGE and Coomassie staining (bottom panel).
C) Mitochondria purified from H1299 cells were incubated with 1 or 4 ug of wt or mutant recombinant GST-p53 (160–318) proteins for 30 minutes and subsequently cross-linked with BMH. BAK monomers and oligomers were detected by immunoblotting using a BAK specific antibody. Arrows indicate BAK oligomers; open arrowheads indicate BAK monomers and asterisk indicates an intra-molecular crosslink seen with BAK monomers treated with BMH. The purity of mitochondrial preparations was detected by immunoblotting with antisera to controls (right panel). Equal input of recombinant proteins was assessed by SDS-PAGE and Coomassie staining (bottom panel). The slight mobility shift evident in the R175H protein reflects usage of a stop codon within the GST vector rather than in the p53 coding sequence.
D) Mitochondria purified from H1299 cells were incubated with 3 ug of wt or mutant recombinant GST-p53 (160–318) proteins for 45 minutes. As a positive control for cytochrome C release 3 mM CaCl2 was added to purified mitochondria. Cytochrome C release from mitochondria was detected by immunoblotting using an antibody specific for cytochrome C. Equal input of recombinant proteins was assessed by SDS-PAGE and Coomassie staining (bottom panel).
E) Saos-2 cells stably transfected with vector or p53 mutated at codons 175, 273, or 277 were treated with camptothecin (CPT) for 5 hours. In parallel, Saos-2 cells stably transfected with temperature sensitive p53 were temperature shifted from 39°C (mutant conformation p53) to 32°C (wild type conformation p53). 300 ug of whole cell lysate was immunoprecipitated with a BAK specific antibody (BAK-NT, Upstate). Immunoprecipitated complexes were captured with protein G agarose beads and subjected to SDS-PAGE and Western blotting with a p53 specific antibody (AB-1, EMD, Biosciences). In the bottom panel, whole cell lysates of cells expressing mutant p53 were subjected to Western blotting with a p53 specific antibody (Ab-1, EMD Biosciences) and an actin specific antibody (Ac-15, Sigma) to confirm equal protein loading.
F) Colony formation assay of Saos-2 cells transfected with an expression vector encoding either mitochondrially targeted wild type (wt) p53 (L-p53 wt) or mutant proteins (L-p53 R175H, L-p53 R273H, and L-p53 C277F), compared to parental vector following selection in G418.
