Abstract
Cellular aging occurs by the lifelong accumulation of oxidative damage leading to neuronal apoptosis, termed ‘neurodegeneration’, and the functional deficits of aging. Loss of visual function is one of the most important quality of life measures for older adults. We discuss recent clinical and laboratory advances in the neuroprotective treatment of the aging eye with particular emphasis on the three major ocular neurodegenerative conditions: glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy (DR).
Keywords: age-related macular degeneration, calcium, diabetic retinopathy, glaucoma, neurodegeneration, neuroprotection, retina
Introduction
Apoptosis, also known as programmed cell death, is a fundamental cellular signaling mechanism relevant to the proper function of a wide range of physiological processes from the single cell to the whole organism. As cells age, however, they accumulate damage from multiple sources, oxidative damage being the main focus of current aging research. A ‘dysregulated’ cell must either be destroyed or reverted to the healthy state in order to preserve the proper function of tissues and even the whole organism. The term ‘neuroprotection’, the protection of nerve cells from acute or chronic damage, should thus not be interpreted to mean solely the support of failing cells but should include the restoration of normal physiology. The goal of neuroprotection must be to prevent cell death by apoptosis or necrosis along with the correction of the physiology causing cellular pathology.
The retina is an ideal target for neuroprotection. Projected from the brain during development, the retina is exposed central nervous system (CNS) tissue that is more readily available for pharmacologic intervention than the remainder of the CNS. The regulated control of chemical access to the eye by the blood-retina barrier and the concomitant immune privilege allow interventions prohibitive elsewhere due to side effects.
Aging is theorized to occur by accumulative damage to the normal function of the cell by reactive oxygen species (ROS), such as free radicals and hydroxyl radical,1 often beginning as small synaptic changes but over time resulting in both synaptic and cellular dysfunction. The retina is a high oxygen demand tissue due to the large amount of energy required to drive visual signal transduction. Oxygen saturation and vascularization decrease towards the inner retinal layers, but compensatory neovascularization in response to ischemia interferes with visual function and is a distinct marker of retinal disease.2 The oxidative load created by normal visual signal transduction is counterbalanced by multiple antioxidant signaling systems, optimized and regulated to function in tandem. The cellular redox potential is actively maintained by antioxidant cascades that converge on the mitochondria. The mitochondrion is the primary metabolic organelle, responsible for producing ATP by the classical glucose pathway, but mitochondria also regulate intracellular pH, cytosolic calcium concentrations, and control cellular signaling resulting in apoptosis through specific enzyme and cellular messenger pathways.3 The majority of intracellular ROS are generated as a byproduct of the mitochondrial respiratory chain through electron leakage from mitochondrial complex I and III leading to the production of superoxide (O2•-).4 Superoxide conversion to hydrogen peroxide (H2O2) is mediated by the scavenger enzymes superoxide dismutase (SOD), specifically the cytosolic Cu/Zn SOD1 and the mitochondrial matrix MnSOD2.4 Hydrogen peroxide can react with reduced iron (Fe2+) to form the hydroxyl radical (•OH), a potent ROS, that binds proteins, catalyzes the formation of lipid peroxyl radicals, and mutates DNA bases by a cyclization reaction.4 Redox biochemistry and its clinical relevance have been recently reviewed by Valko and colleagues.4
Neuroprotective approaches have shown great promise in in vitro and in vivo studies. For instance, N-acylethanolamines (NAEs) are a class of signaling lipids endogenously expressed widely in the CNS 5, including the retina.6 Several NAE species and NAE precursor molecules are up-regulated in response to chemical and traumatic insults, a cellular ability that decreases with age. This suggests a role in neuroprotection. 5,7
NAEs such as NAE 20:4 (arachidonylethanolamine; AEA) bind cannabinoid receptors (CB1 and CB2), coining the term ‘endocannabinoids’.5 However, recent experimental evidence indicates that the neuroprotective effects of NAEs are not mediated via the cannabinoid receptor system.7, 8 Several NAEs (NAE 16:0, NAE 12:0, and NAE 18:2) have been shown to reduce stroke volume and improve behavioral outcomes in the rat middle cerebral artery occlusion stroke model (Figs. 1, 2).7,9 Inhibitors for CB1 and TRPV1 receptors did not affect neuroprotection, while the cannabinoid uptake inhibitor AM404 blocked NAE-mediated neuroprotection.7 Similar results were obtained from in vitro studies, which suggest that neuroprotection is mediated through an intracellular mechanism, likely resulting in reduction of oxidative stress and glutamate excitotoxicity through the modulation of intracellular calcium homeostasis.8,10 Of particular relevance, NAE 18:2 has potent neuroprotective effects against glutamate cytotoxicity in ex vivo retinal explants (Figs. 3, 4).11
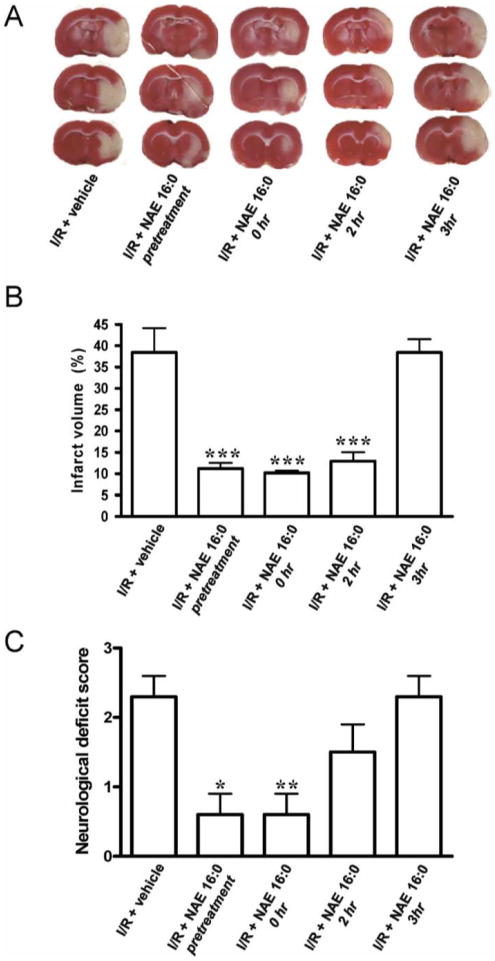
Figure 1. NAE 16:0 is neuroprotective against ischemic tissue damage and improves neurological outcome following I/R.
(A) Representative TTC-stained sections of rat brain following 90 min. MCAO / 24 h reperfusion (ischemia/reperfusion; I/R), treated with vehicle or NAE 16:0 at various time points. Viable tissue stains red, whereas damaged ischemic brain tissue appears unstained/white. (B) Quantification of the volume of the ischemic lesion (infarct volume) revealed that NAE 16:0 was neuroprotective when administered from 30 min. before MCAO (pretreatment), up to two h after occlusion. Administration of NAE 16:0 at 3 h post-MCAO had no effect on the size of the ischemic lesion. (C) I/R causes a moderate to severe neurological phenotype, as determined by the standardized neurological deficit score. NAE 16:0 reduced I/R-induced neurological deficits significantly (none to mild neurological phenotype) when administered before or at the time of occlusion. Data are shown as mean ± s.e.m. * p<0.05, ** p<0.01, *** p<0.001, compared with vehicle treated control group as determined using one-way ANOVA with Newman Keuls multiple comparison post-hoc test.
Reprinted from reference 9, Garg P, Duncan RS, Kaja S, Zabaneh A, Chapman KD, Koulen P. Lauroylethanolamide and linoleoylethanolamide improve functional outcome in a rodent model for stroke. Neurosci Lett. Apr 4 2011;492(3):134-138, with permission from Elsevier Ltd.; permission conveyed through Copyright Clearance Center, Inc. Further reproduction prohibited without permission.
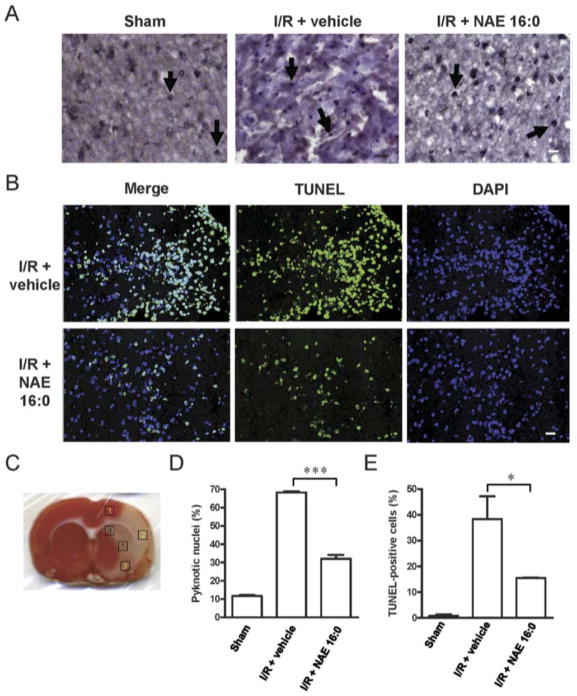
Figure 2. NAE 16:0 protects from I/R-induced apoptosis.
(A) Representative photomicrographs of hematoxilin/eosin (HE) staining within the ischemic area of the cortex in sham-operated and ischemicreperfusion rats with or without NAE 16:0 treatment. Yellow arrows indicate pyknotic nuclei, indicative of cells undergoing apoptosis. Fewer pyknotic nuclei were observed following I/R in the presence of NAE 16:0. Scale bar: 100 μm. (B) TUNEL labeling revealed a substantially reduced number of TUNEL-positive cells in the presence of NAE 16:0. In sham-operated animals, almost no TUNEL-positive cells were identified (data not shown). Scale bar: 50 μm. (C) For quantification of pyknotic nuclei and TUNEL-positive cells, five areas in each section were analyzed. Areas are indicated here on a TTC-stained section of a vehicle-treated rat following I/R. Areas 1-3 are cortical and lie in the ischemic core and penumbral zone, whereas areas 4-5 represent subcortical areas mainly falling in the ischemic penumbral zone. (D) Treatment with NAE 16:0 reduced the number of pyknotic nuclei following MCAO/reperfusion significantly by 53%, compared with vehicle-treated control. (E) Similarly, the number of TUNEL-positive cells was 60% lower in NAE 16:0 treated brain sections, compared with vehicle control, indicating that NAE 16:0 treatment protects from induction of apoptosis and cell-death pathways. Data are presented as mean ± s.e.m. for each group. * p<0.05, *** p<0.001, using one-way ANOVA with Newman Keuls multiple comparison post-hoc test.
Reprinted from reference 9, Garg P, Duncan RS, Kaja S, Zabaneh A, Chapman KD, Koulen P. Lauroylethanolamide and linoleoylethanolamide improve functional outcome in a rodent model for stroke. Neurosci Lett. Apr 4 2011;492(3):134-138, with permission from Elsevier Ltd.; permission conveyed through Copyright Clearance Center, Inc. Further reproduction prohibited without permission.
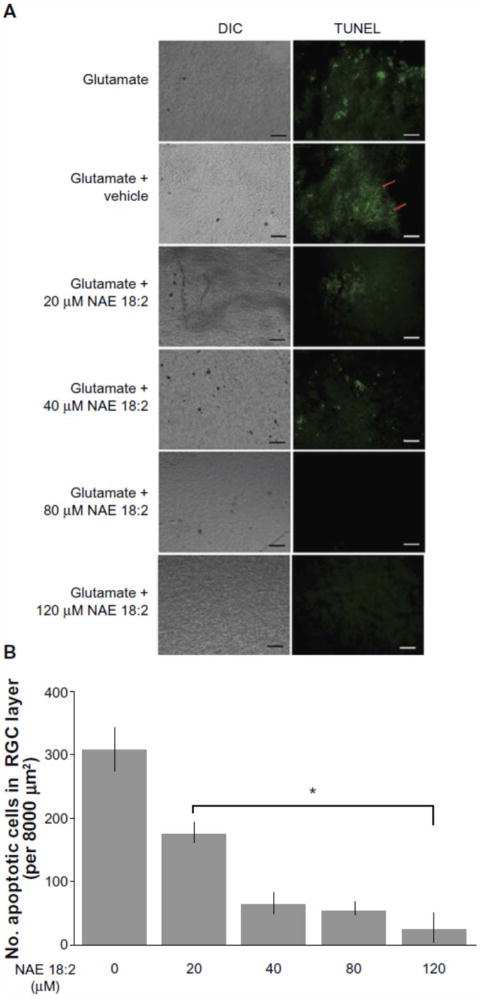
Figure 3. NAE 18:2 protects RGC layer neurons from glutamate excitotoxicity.
A) Exposure of retinas to 100 μM glutamate resulted in a dramatic increase in RGC layer neuron death (red arrows). Preincubation of retinas with NAE 18:2 for 6 hours prior to glutamate exposure resulted in a dosedependent decrease in the number apoptotic, TUNEL-positive RGC layer neurons, with high physiological concentrations reducing the number of apoptotic RGCs. B) Quantitative summary data for the NAE 18:2-mediated neuroprotection from glutamate toxicity as measured by TUNEL histochemistry.
Notes: * denotes P < 0.05 as determined by a one-way analysis of variance test. Scale bar: 50μm.
Abbreviations: DIC, differential interference contrast; NAE, N-acylethanolamide; RGC, retinal ganglion cell; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP (2′-deoxyuridine 5′-triphosphate) nick-end labeling – green fluorescence.
Republished with permission of Dove Medical Press Limited, from reference 11, Duncan RS, Xin H, Goad DL, Chapman KD, Koulen P. Protection of neurons in the retinal ganglion cell layer against excitotoxicity by the N-acylethanolamine, N-linoleoylethanolamine. Clin Ophthalmol. 2011;5:543-548; permission conveyed through Copyright Clearance Center, Inc. Further reproduction prohibited without permission.
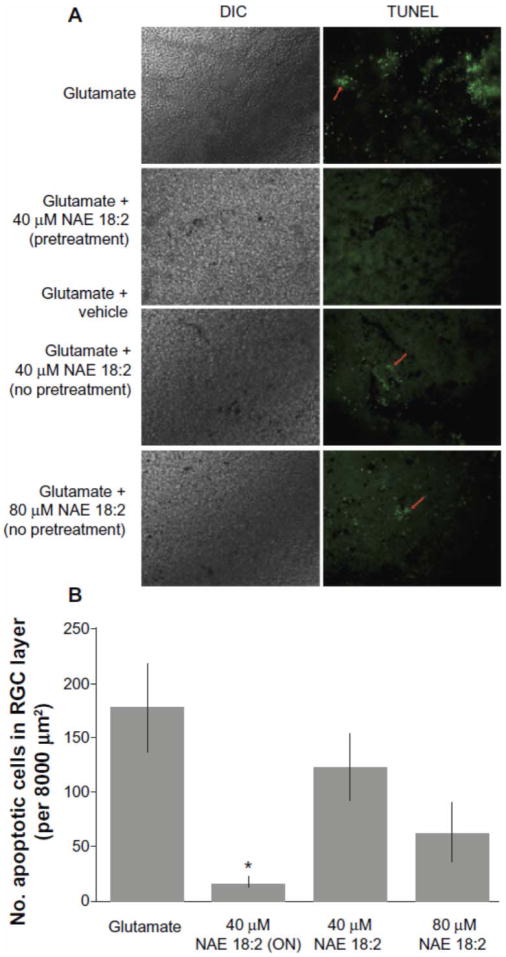
Figure 4. NAE 18:2 preincubation is required for protection of RGC layer neurons against glutamate excitotoxicity.
NAE 18:2 preincubation is critical for its neuroprotective effect. A), Retina explants were either pretreated for 24 hours (overnight, ON) with 40 μM NAE 18:2 prior to and during glutamate exposure or at the onset of glutamate exposure (no pretreatment). All glutamate exposures were for 24 hours and NAE 18:2 was present during the glutamate exposure period. Note the lack of protection without pretreatment (red arrows). B) Quantitative summary data revealing the requirement for NAE 18:2 protection of RGC layer neurons against glutamate.
Notes: * denotes P < 0.05 as determined by a one-way analysis of variance test. Scale bar: 50μm.
Abbreviations: DIC, differential interference contrast; NAE, N-acylethanolamide; RGC, retinal ganglion cell; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP (2′-deoxyuridine 5′-triphosphate) nick-end labeling – green fluorescence.
Republished with permission of Dove Medical Press Limited, from reference 11, Duncan RS, Xin H, Goad DL, Chapman KD, Koulen P. Protection of neurons in the retinal ganglion cell layer against excitotoxicity by the N-acylethanolamine, N-linoleoylethanolamine. Clin Ophthalmol. 2011;5:543-548; permission conveyed through Copyright Clearance Center, Inc. Further reproduction prohibited without permission.
Non-cannabinoid acting NAEs, such as NAE 16:0 and NAE 18:2, lack the acute and long-term chronic side-effects associated with cannabinoid use 6 and provide a safe alternative for neuroprotective approaches for disorders of the retina. Additional in vivo studies are warranted to establish the feasibility of therapeutic intervention strategies involving NAEs in glaucoma and other neurodegenerative disorders of the eye.
Oxidative damage causes a profound dysregulation of cellular physiology and contributes to the pathophysiology of neurodegenerative disorders such as glaucoma, diabetic retinopathy (DR), and age-related macular degeneration (AMD). The present review will discuss some recent advances in neuroprotective strategies developed to counter oxidative damage in these three major ophthalmic diseases.
Glaucoma
Glaucoma is a neuropathy of the aging eye primarily characterized by increased intraocular pressure (IOP) and eventual loss of visual function by destruction of the optic nerve and retinal ganglion cells (RGCs); the most common form is primary open angle glaucoma. 12 It should be noted that a significant minority of glaucoma patients present without increased IOP (i.e. normal tension glaucoma) and that glaucoma can also occur early in life or resulting from traumatic injury. However, IOP is the only clinically treatable modality and is the most predictive risk factor.12 Debate exists over the causative role of IOP in disease progression, though the primary treatment endpoint for glaucoma remains the decrease of IOP by at least 25% from the patient’s baseline untreated IOP.13 The order of disease progression of glaucoma, whether the death of RGCs presage or result from the degeneration of the optic nerve is an interesting topic but beyond the scope of this review. 12
The canonical treatment for the reduction of IOP in the most common form of glaucoma, primary open angle glaucoma,13 remains the topical use of β receptor antagonists or prostaglandin analogues, first separately but in combination if IOP does not decrease within one year following the initiation of treatment.14 Common alternate treatment modalities include carbonic anhydrase inhibitors or α2 adrenergic agonists. 13,14 Many different treatment paradigms have been tested in recent years in an effort to preserve visual function by sustaining the RGCs. Initially, the most promising drug in clinical trials was memantine, an N-methyl-D-aspartate (NMDA) channel antagonist with micromolar affinity. Memantine binds the open NMDA channel, blocking calcium entry to the cell or synapse, which should be neuroprotective in cases of glutamate excitotoxicity.15 Indeed, memantine is neuroprotective in laboratory tests with rodents 16 but has failed to protect RGCs in primate studies. 17,18 Memantine has been shown to alter structural parameters of the eye and retina,19 but these data are not not clearly linked to any measurable functional effects. Repeated, expensive phase III clinical trials failed to find an indication for memantine in neuroprotection of RGCs or the treatment of glaucoma.20 A related compound, bis(7)-tacrine, has been discussed as a possible successor to memantine due to its improved efficacy in laboratory tests of RGC survival but it has yet to reach clinical trials.21
Recent studies have shown that the well-known surgical and dental dye, methylene blue, has a unique mechanism of neuroprotection. In this novel mechanism of neuroprotection, methylene blue accepts electrons from NADH early in the mitochondrial electron transport complex and shuttles the electrons past complex I and III to cytochrome C.22 The methylene blue alternative pathway to the electron transport chain reduced production of superoxide and free radicals in response to physiological oxidative stress induced by glutamate, ischemia, or electron transport complex specific inhibitors but not to direct chemical oxidation by hydrogen peroxide or glucose oxidase.22,23 The electron scavenging ability of methylene blue was neuroprotective in cultured rat RGCs exposed to mitochondria electron transport complex inhibitors rotenone and staurosporine.24 Methylene blue has yet to enter clinical trials for neuroprotection but its safety is known due to its past use as an antimalarial agent. Furthermore, its high bioavailability from oral dosage makes it a potentially powerful systemic neuroprotectant.25
Direct control of the calcium flux into the cell by use of L-type calcium channel blockers may be neuroprotective in the retina.26 Though their clinical use is decreased due to systemic side effects, calcium channel blockers acting on the plasma membrane calcium channels decrease the cytosolic calcium concentration. Elevation of cytosolic calcium is causative of many neuronal pathologies and leads to cellular dysregulation, oxidative stress, and apoptosis.27 In glaucoma, calcium channel blockers have an indication for the dilation of retinal blood vessels to increase fluid drainage and decrease IOP.26 The β blocker nimodipine has been clinically demonstrated to have a positive effect on the visual field 28, increase contrast sensitivity 29, and prevent RGC loss by the inhibition of extracellular calcium influx. Ex vivo studies of calcium channel blockers demonstrated that nimodipine, iganidipine, and lomerizine were neuroprotective against hypoxic insult in cultured RGCs.30 Previous clinical trials for calcium channel blockers in ophthalmology have rightfully focused their endpoints on the IOP and ocular blood flow, but clinical trials with neuroprotective endpoints, including relevant functional tests, for calcium channel antagonists would be required to correlate the promising laboratory data with clinical utility.
Age related macular degeneration (AMD)
AMD is a common, multifactorial eye disease caused by an interaction of lifetime accumulated retinal damage and potentially also by genetic factors. Diagnostic guidelines for the ‘dry’ form of AMD require identification of intermediate to large deposits called drusen (>125 microns), autofluorescent lipofuscin granules, patchy loss of retinal pigment epithelium (RPE), and, in the aptly named ‘wet’ form of AMD, choroidal neovascularization.31 AMD clinical pathology has been thoroughly described 32 and the genetic susceptibilities for AMD have recently been reviewed.33 The progressive neovascularization in wet AMD, which is observed in about 15% of all AMD cases, usually responds well to treatment with inhibitors of vascular endothelial growth factor (VEGF).31 Intravitreal injection of the corticosteroid triamcinolone acetonide has been proposed but conflicting reports on efficacy, and frequent reports of side effects with only marginal reduction of vision loss, decrease its clinical utility.31,34
Not surprisingly, since ‘aging’ is literally included in the name of the disease, cumulative lifetime oxidative stress is the presumptive mechanism of neurodegeneration. Completion of the Age-Related Eye Disease Study (AREDS) of dietary supplementation with antioxidants in AMD patients has yielded marginally positive results slowing disease progression after the intermediate stage, though longer trial endpoints (>5 years) will be needed to properly verify that claim.31 Polyphenol compounds of certain green teas and their extracts are potentially neuroprotective in the photoreceptor outer segment and RPE if delivered in the proper dosages, namely about two cups of green tea per day.35 Genetic knockdown of either cytosolic Cu/Zn SOD136 or mitochondrial MnSOD237 in mice increased markers of oxidative stress in the retina and recapitulated AMD phenotype with increased drusen, thickening of Bruch’s membrane, and degeneration of the RPE and photoreceptor layers. Conversely, overexpression of MnSOD2 was neuroprotective against oxidative stress and decreased the AMD phenotype.38 Recent studies have described a novel signaling cycle where generation of ROS by cytoplasmic NADPH oxidase triggers increased superoxide production in the mitochondria beyond the saturation point of MnSOD2.39 Diffusion of superoxide to the cytosol activates NADPH oxidase to generate more ROS and promotes a vicious cycle of oxidative damage.39 Relevant to both AMD and DR, increased activity of NADPH oxidase has been found to increase angiogenesis, vascular damage by leukocyte adhesion, and accumulation of advanced glycation end-products.35
Serotonin receptor 1A (5-HT1A) agonists have recently demonstrated neuroprotective properties in the retina although the mechanism is unclear. Topical treatment with the 5-HT1A agonists 8-OH DPAT and AL-8309 prevented damage to the photoreceptor layer and RPE when applied prior to hydrogen peroxide treatment in vitro40 or phototoxic insult in vivo41, respectively. Phototoxic damage proceeds from the singlet oxygen, superoxide, and hydrogen peroxide generated by blue light excitation of bisretinoids, compounds in lipofuscin granules of the photoreceptor outer segment.42 The process of formation of lipofuscin granules is not clear but they seem to be photoreceptor outer segments incompletely digested by the RPE.35,42 Serotonin agonists were also found to lessen the immune system aspect of drusen and lipofuscin deposition through a decrease in the recruitment of leukocytes, microglia, and certain proteins of the complement pathway.43
Diabetic Retinopathy (DR)
DR is a common effect of diabetes with a 90% risk of onset within 25 years of diabetes diagnosis.44 Neurodegeneration in DR is presaged by vascular degeneration in response to both the metabolic abnormalities and systemic high glucose concentration encountered in diabetes. Elevated blood glucose concentrations cause pericyte cell death, thickening of the capillary basement membrane, and accumulation of advanced glycation end-products.44 Hard, yellow exudates accumulate from epithelia proliferation at the site of pericyte loss which leads to decreased blood flow and patches of ischemic damage in the microvascular circuit and surrounding neuronal cells. Ischemic lesions are the first clinically detectable symptom of non-proliferative DR.44 The patient may advance to proliferative DR and more extensive damage caused by neovascularization, a compensatory response to the hypoxic conditions of non-proliferative DR.44 During neovascularization, fragile new blood vessels connecting to the vitreous are stimulated to grow but fail to survive, causing scaring in the vitreous body and often leading to very poor vision or even blindness. Currently, the preferred treatment for DR is tight control of blood glucose but this often only delays retinal neurodegeneration.45 Further treatments vary by severity and stage of DR progression but both laser photocoagulation and vitrectomy are invasive although both have decent outcomes.45 Neovascularization can be slowed or blocked by inhibitors of VEGF.45
Dietary supplementation with antioxidants such as trolox, N-acetyl cysteine, and vitamins E and C has been suggested for DR but, as in AMD, the clinical trial outcomes are not overly encouraging due to similar experimental design concerns.46 Green tea polyphenols such as (-)-epigallocatechin gallate are neuroprotective by redox pathways in animal studies of the brain 47 and retina.48 However, green tea extracts have been demonstrated to increase glucose crosslinking49, a neurodegenerative effect of elevated blood glucose concentrations, therefore patients should be cautioned towards moderation in the use of green teas. Dietary supplementation with zeaxanthin, a photosensitive carotenoid concentrated in the retina, has shown some efficacy at preventing induction of VEGF and inhibition of the inflammatory immune response to DR.50 Pharmacological neuroprotective treatments are possible in cases of DR but are retarded by patient non-compliance with glucose control, therefore the presumptive neuroprotective strategy would likely require prior and ongoing correction of blood glucose.
Conclusion
Retinal neuroprotection is a rapidly advancing field, full of interesting challenges and therapeutic potential. Neuroprotective treatment must first halt the apoptotic or necrotic death of post-mitotic cells and, in most cases, ideally arrest the advancement of disease. The second goal of a neuroprotective treatment is the reversion of pathologic cellular function to the healthy physiologic state. This second goal may be unattainable in some cases but often the inhibition of disease progression is contingent upon reversion to normal cellular function. Novel treatments targeting newly discovered neuroprotective pathways will enter clinical trials in the coming years and patients’ lives will be improved by remission or correction of the pathologies of disease and aging. Neuroprotective strategies have been successful both in vitro and in vivo at halting or reversing damage to aged neurons. Clinical trials with dietary antioxidant supplementation have not produced an indication for neurodegenerative disease likely due to the choice of study endpoints or independent variables such as patient lifestyle and disease stage. Since retina pathologies tend to be multifactorial, neuroprotective treatments may be feasible as a component of treatment regimens that address multiple symptoms. For example, in glaucoma, where pharmacologic treatments exist to lower IOP, neuroprotective compounds that address the degeneration of the optic nerve or death of RGCs may aid in reversal of the multifactorial pathology.
Patients should be advised of the risk of pro-oxidative behaviors such as excessive exercise 51 or smoking.35 The advancements discussed above will begin or have begun the translation to the clinic but some precautions can be widely advised to use antioxidant supplementation with polyphenols, readily available in green teas, or dietary restriction, such as tight blood glucose control and general caloric restriction. Antioxidant treatments for neuroprotection do involve some risk since many antioxidant compounds are capable of becoming pro-oxidants at high concentrations. Patients should be reminded of the carving in the lintel of the Delphic Temple of Apollo, μηδὲν ἄγαν - “Nothing excessively,” that is still sage council.
Acknowledgments
Research reported in this publication was supported in part by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, the Vision Research Foundation of Kansas City, and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged. The authors thank Margaret, Richard and Sara Koulen for generous support and encouragement.
Table of Abbreviations
- 5-HT1A
serotonin receptor 1A
- AREDS
Age-Related Eye Disease Study
- AMD
age related macular degeneration
- CB
cannabinoid receptor
- CNS
central nervous system
- DR
diabetic retinopathy
- IOP
intraocular pressure
- RGC
retinal ganglion cell
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- SOD
superoxide dismutase
- VEGF
vascular endothelial growth factor
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Lange CA, Bainbridge JW. Oxygen sensing in retinal health and disease. Ophthalmologica. 2012;227(3):115–131. doi: 10.1159/000331418. [DOI] [PubMed] [Google Scholar]
- 3.Osborne NN, del Olmo-Aguado S. Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr Opin Pharmacol. 2013 Feb;13(1):16–22. doi: 10.1016/j.coph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Fride E. Endocannabinoids in the central nervous system--an overview. Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):221–233. doi: 10.1054/plef.2001.0360. [DOI] [PubMed] [Google Scholar]
- 6.Yazulla S. Endocannabinoids in the retina: from marijuana to neuroprotection. Prog Retin Eye Res. 2008 Sep;27(5):501–526. doi: 10.1016/j.preteyeres.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg P, Duncan RS, Kaja S, Koulen P. Intracellular mechanisms of N-acylethanolamine-mediated neuroprotection in a rat model of stroke. Neuroscience. 2010 Mar 10;166(1):252–262. doi: 10.1016/j.neuroscience.2009.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombardi G, Miglio G, Varsaldi F, Minassi A, Appendino G. Oxyhomologation of the amide bond potentiates neuroprotective effects of the endolipid N-palmitoylethanolamine. J Pharmacol Exp Ther. 2007 Feb;320(2):599–606. doi: 10.1124/jpet.106.112987. [DOI] [PubMed] [Google Scholar]
- 9.Garg P, Duncan RS, Kaja S, Zabaneh A, Chapman KD, Koulen P. Lauroylethanolamide and linoleoylethanolamide improve functional outcome in a rodent model for stroke. Neurosci Lett. 2011 Apr 4;492(3):134–138. doi: 10.1016/j.neulet.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan RS, Chapman KD, Koulen P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Mol Neurodegener. 2009;4:50. doi: 10.1186/1750-1326-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan RS, Xin H, Goad DL, Chapman KD, Koulen P. Protection of neurons in the retinal ganglion cell layer against excitotoxicity by the N-acylethanolamine, N-linoleoylethanolamine. Clin Ophthalmol. 2011;5:543–548. doi: 10.2147/OPTH.S18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012 Nov;31(6):702–719. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern Guidelines. San Francisco, CA: American Academy of Ophthalmology; 2010. Primary Open-Angle Glaucoma. [Google Scholar]
- 14.Boland MV, Ervin AM, Friedman DS, et al. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2013 Feb 19;158(4):271–279. doi: 10.7326/0003-4819-158-4-201302190-00008. [DOI] [PubMed] [Google Scholar]
- 15.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2004 Jan;1(1):101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1618–1624. [PubMed] [Google Scholar]
- 17.Hare W, WoldeMussie E, Lai R, et al. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv Ophthalmol. 2001 May;45(Suppl 3):S284–289. doi: 10.1016/s0039-6257(01)00200-4. discussion S295-286. [DOI] [PubMed] [Google Scholar]
- 18.Hare WA, WoldeMussie E, Lai RK, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Invest Ophthalmol Vis Sci. 2004 Aug;45(8):2625–2639. doi: 10.1167/iovs.03-0566. [DOI] [PubMed] [Google Scholar]
- 19.Hare WA, WoldeMussie E, Weinreb RN, et al. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, II: Structural measures. Invest Ophthalmol Vis Sci. 2004 Aug;45(8):2640–2651. doi: 10.1167/iovs.03-0567. [DOI] [PubMed] [Google Scholar]
- 20.Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009 Jun;87(4):450–454. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 21.Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC neuroscience. 2010;11:31. doi: 10.1186/1471-2202-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Y, Li W, Poteet EC, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011 May 6;286(18):16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poteet E, Winters A, Yan LJ, et al. Neuroprotective actions of methylene blue and its derivatives. PloS one. 2012;7(10):e48279. doi: 10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daudt DR, 3rd, Mueller B, Park YH, Wen Y, Yorio T. Methylene blue protects primary rat retinal ganglion cells from cellular senescence. Invest Ophthalmol Vis Sci. 2012 Jul;53(8):4657–4667. doi: 10.1167/iovs.12-9734. [DOI] [PubMed] [Google Scholar]
- 25.Walter-Sack I, Rengelshausen J, Oberwittler H, et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. European journal of clinical pharmacology. 2009 Feb;65(2):179–189. doi: 10.1007/s00228-008-0563-x. [DOI] [PubMed] [Google Scholar]
- 26.Araie M, Mayama C. Use of calcium channel blockers for glaucoma. Prog Retin Eye Res. 2011 Jan;30(1):54–71. doi: 10.1016/j.preteyeres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Duncan RS, Goad DL, Grillo MA, Kaja S, Payne AJ, Koulen P. Control of intracellular calcium signaling as a neuroprotective strategy. Molecules. 2010 Mar;15(3):1168–1195. doi: 10.3390/molecules15031168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piltz JR, Bose S, Lanchoney D. The effect of nimodipine, a centrally active calcium antagonist, on visual function and mascular blood flow in patients with normal-tension glaucoma and control subjects. J Glaucoma. 1998 Oct;7(5):336–342. [PubMed] [Google Scholar]
- 29.Bose S, Piltz JR, Breton ME. Nimodipine, a centrally active calcium antagonist, exerts a beneficial effect on contrast sensitivity in patients with normal-tension glaucoma and in control subjects. Ophthalmology. 1995 Aug;102(8):1236–1241. doi: 10.1016/s0161-6420(95)30884-6. [DOI] [PubMed] [Google Scholar]
- 30.Yamada H, Chen YN, Aihara M, Araie M. Neuroprotective effect of calcium channel blocker against retinal ganglion cell damage under hypoxia. Brain research. 2006 Feb 3;1071(1):75–80. doi: 10.1016/j.brainres.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern Guidelines. San Francisco, CA: American Academy of Ophthalmology; 2008. Age-Related Macular Degeneration. [Google Scholar]
- 32.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003 May-Jun;48(3):257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 33.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009 Jan;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maberley D. Canadian Retinal Trials G. Photodynamic therapy and intravitreal triamcinolone for neovascular age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2009 Nov;116(11):2149–2157. e2141. doi: 10.1016/j.ophtha.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012 Aug;33(4):399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imamura Y, Noda S, Hashizume K, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006 Jul 25;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justilien V, Pang JJ, Renganathan K, et al. SOD2 knockdown mouse model of early AMD. Invest Ophthalmol Vis Sci. 2007 Oct;48(10):4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasahara E, Lin LR, Ho YS, Reddy VN. SOD2 protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3426–3434. doi: 10.1167/iovs.05-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dikalova AE, Bikineyeva AT, Budzyn K, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circulation research. 2010 Jul 9;107(1):106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thampi P, Rao HV, Mitter SK, et al. The 5HT1a receptor agonist 8-Oh DPAT induces protection from lipofuscin accumulation and oxidative stress in the retinal pigment epithelium. PloS one. 2012;7(4):e34468. doi: 10.1371/journal.pone.0034468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier RJ, Patel Y, Martin EA, et al. Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest Ophthalmol Vis Sci. 2011 Apr;52(5):2118–2126. doi: 10.1167/iovs.10-6304. [DOI] [PubMed] [Google Scholar]
- 42.Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012 Mar;31(2):121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collier RJ, Wang Y, Smith SS, et al. Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT1A agonist. Invest Ophthalmol Vis Sci. 2011;52(11):8108–8116. doi: 10.1167/iovs.10-6418. [DOI] [PubMed] [Google Scholar]
- 44.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008 Dec;9(4):315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 45.American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern Guidelines. San Francisco, CA: American Academy of Ophthalmology; 2008. Diabetic Retinopathy. [Google Scholar]
- 46.Kowluru RA, Zhong Q. Beyond AREDS: is there a place for antioxidant therapy in the prevention/treatment of eye disease? Invest Ophthalmol Vis Sci. 2011 Nov;52(12):8665–8671. doi: 10.1167/iovs.10-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain research. 2004 Sep 3;1019(1-2):47–54. doi: 10.1016/j.brainres.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 48.Silva KC, Rosales MA, Hamassaki DE, et al. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(2):1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 49.Mustata GT, Rosca M, Biemel KM, et al. Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats: improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes. 2005 Feb;54(2):517–526. doi: 10.2337/diabetes.54.2.517. [DOI] [PubMed] [Google Scholar]
- 50.Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 51.Camiletti-Moiron D, Aparicio VA, Aranda P, Radak Z. Does exercise reduce brain oxidative stress? A systematic review. Scandinavian journal of medicine & science in sports. 2013 Mar 15; doi: 10.1111/sms.12065. [DOI] [PubMed] [Google Scholar]