Table 1.
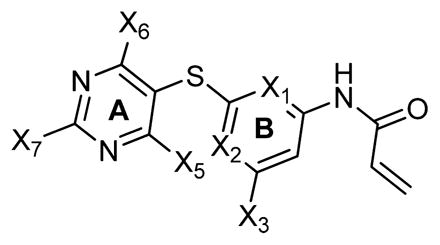
| ||||||
|---|---|---|---|---|---|---|
| compd | X1 = X2 | X3 | X5, X6 | X7 | IC50 (μM) growth inhibitiona | IC50 (μM) caspase-3,7 activationb |
| 17a | N | -NH2 | 2 × -OCH3 | methylpiperazine | 0.90 ± 0.2 | 1.2 ± 0.4 |
| 17b | N | -NH2 | 2 × -OCH3 | morpholine | 12.5 ± 0.7 | 12.1 ± 2.5 |
| 17c | N | -NH2 | 2 × -OCH3 | piperidine | 17.1 ± 2.2 | 16.2 ± 0.5 |
| 20a | N | -NH2 | 2 × -OC2H5 | methylpiperazine | 0.89 ± 0.2 | 4.0 ± 0.0 |
| 20b | N | -NH2 | 2 × -OC2H5 | morpholine | 2.2 ± 1.2 | 10.5 ± 3.5 |
| 20c | N | -NH2 | 2 × -OC2H5 | piperidine | 4.5 ± 0.2 | 4.0 ± 2.0 |
| 27a | N | -H | 2 × -OCH3 | methylpiperazine | 1.25 ± 0.7 | 4.0 ± 0.5 |
| 27b | N | -H | 2 × -OCH3 | morpholine | 2.0 ± 0.5 | 7.2 ± 0.7 |
| 27c | N | -H | 2 × -OCH3 | piperidine | 3.15 ± 1.5 | 12.4 ± 1.5 |
| 27d | N | -H | 2 × -OCH3 | pyrrolidine | 46.1 ± 15.5 | >100 |
| 28 | N | -H | 2 × -OCH3 | 1-methylpiperazine 1-oxide | >100 | >100 |
| 30 | N | -H | 2 × -OCH3 | -OCH3 | ND | 7.5 ± 0.3 |
| 31 | N | -NH2 | 2 × -OCH3 | -NH2 | 3.9 ± 1.4 | 4.5 ± 0.5 |
| 36a | C | -H | 2 × -OCH3 | methylpiperazine | 7.8 ± 1.1 | ND |
| 37 | C | -H | 2 × -OCH3 | morpholine | 46.5 ± 3.4 | 27.5 ± 2.5 |
| 42a | N | -H | 2 × -CH3 | methylpiperazine | ND | 6.0 ± 2.1 |
| 43 | N | -H | 2 × -CH3 | morpholine | 14.4 ± 2.7 | 15.4 ± 0.7 |
| 44a | N | -NH2 | 2 × -OCH3 | 2-(2-(2-(2-(piperazin-1-yl)ethoxy)ethoxy) ethoxy) ethanol | 3.8 ± 1.2 | 14.3 ± 1.5 |
| 45a | N | -NH2 | -OCH3, -(OC2H4O)4H | methylpiperazine | 76.5 ± 21.5 | 100 ± 10.5 |
Inhibition of growth measured in Kasumi-1 acute myeloid leukemia cells. Values are the mean ± SEM.
Caspase-3,7 activation measured in MOLM13 acute myeloid leukemia cells. Values are the mean ± SEM.
