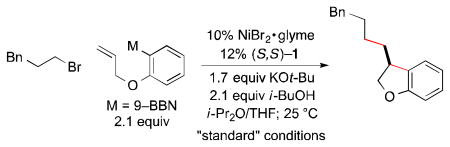
Table 1. Catalytic Enantioselective Cyclization/Cross-Coupling with an Alkyl Electrophile: Influence of Reaction Parametersa.
| |||
|---|---|---|---|
| entry | variation from the “standard” conditions | ee (%) | yield (%)b |
| 1 | none | 96 | 82 |
| 2 | no NiBr2 • glyme | – | <5 |
| 3 | no (S,S)–1 | – | <5 |
| 4 | no i-BuOH | – | <5 |
| 5 | 1.5 equiv of arylboron reagent | 81 | 67 |
| 6 | (S,S)–2, instead of (S,S)–1 | 39 | 64 |
| 7 | (S,S)–3, instead of (S,S)–1 | – | <5 |
| 8 | (S,S)–4, instead of (S,S)–1 | 61 | 33 |
| 9 | BnCH2CH2Cl, instead of BnCH2CH2Br | – | <5 |
|
| |||
All data are the average of two experiments.
The yield was determined by GC analysis with the aid of a calibrated internal standard.
